LEE, CHO, and KIM: Genome size estimation of 43 Korean Carex
Abstract
The genome size is defined as the amount of DNA in an unreplicated gametic chromosome complement and is expressed as the 1C value. It is a fundamental parameter of organisms that is useful for studies of the genome, as well as biodiversity and conservation. The genome sizes of Korean plants, including Carex (Cyperaceae), have been poorly reported. In this study, we report the genome sizes of 43 species and infraspecific taxa of Korean Carex using flow cytometry, and these results represent about 24.4% of the Carex species and infraspecific taxa distributed on the Korean peninsula. The Plant DNA C-Value Database (release 7.1) updated with and now including our data (a total of 372 Carex accessions) shows that the average genome size of members of the Carex species is 0.47 pg (1C), and the largest genome (C. cuspidate Bertol.; 1C = 1.64 pg) is 8.2 times larger than the smallest (C. brownii Tuck., C. kobomugi Ohwi, C. nubigena D. Don ex Tilloch & Taylor, and C. paxii Kük.; 1C = 0.20 pg). The large genomes are frequently found in the subgen. Carex, especially in sect. Aulocystis, sect. Digitatae, sect. Glaucae, sect. Paniceae, and sect. Siderostictae. Our data updates the current understanding of genome sizes in Carex. This will serve as the basis for understanding the phylogeny and evolution of Carex and will be especially useful for future genome studies.
Keywords: Carex, C-value, Cyperaceae, genome size, flow cytometry
The 1C-value is defined as the amount of DNA in an unreplicated gametic chromosome complement ( Bennett and Leitch, 2011) and it is a key characteristic of organisms and one that is useful for studies of biodiversity, taxonomy, ecology, phylogeny, and population genetics. Recently, the importance of measuring genome size has been particularly emphasized in whole genome sequencing efforts, because the genome size of a taxon is directly related to the efficiency of its genome sequencing. Selecting a species having the smallest genome size in the target plant group can increase the depth of the sequencing result in next-generation sequencing (NGS) and in addition can minimize the computer memory required for genome assembly. As a preliminary step in genome research, a survey of the genome size of the targeted species and related taxa is essential. The Plant DNA C-value Database (release 7.1; http://data.kew.org/cvalues/) managed by the Royal Botanic Gardens, Kew, currently contains 12,273 species comprising 10,770 angiosperms, 421 gymnosperms, 303 pteridophytes (246 ferns and fern allies and 57 lycophytes), 334 bryophytes, and 445 algae ( Zonneveld et al., 2005; Leitch et al., 2019) and these numbers have grown since Bennett and Smith (1976) first published compiling lists of DNA C-values in angiosperms. The genome size of angiosperms varies enormously, with the largest genome being from Paris japonica (Franch. & Sav.) Franch. (Melanthiaceae) (152.23 pg) ( Pellicer et al., 2010) while the smallest is from Genlisea margaretae Hutch. (Lentibulariaceae) (0.061 pg) ( Fleischmann et al., 2014). Huge genome size variations in angiosperms are probably due to the polyploidization and the replication of transposable elements (TEs) ( Grover and Wendel, 2010; Kejnovsky et al., 2012; Leitch and Leitch, 2012). The genus Carex L. (Cyperaceae) is the largest genus in the temperate zone having approximately 2,000 species worldwide ( Global Carex Group, 2015). This genus is characterized by unisexual flowers without perianth and the perigynium in pistillate flowers, which is a specialized floral organ placed outside of a carpel ( Global Carex Group, 2015).
Carex is a plant group having holocentric chromosomes that might cause relatively rapid speciation ( Hipp et al., 2010; Chung et al., 2012; Escudero et al., 2012). Holocentric chromosomes are characterized by a diffuse centromere that facilitates fission (agmatoploidy), fusion (symploidy), translocations, and inversion ( Hipp et al., 2013). Therefore, chromosome numbers in Carex exhibit high diversity ranging from n = 6 to n = 66 ( Tanaka, 1949; Hipp et al., 2009; Chung et al., 2016). To date, the genome size of Carex species has been reported for 329 accessions (292 species and infraspecific taxa; 276 species) ranging from 1C = 0.2 pg ( C. brownii Tuck., C. kobomugi Ohwi, C. nubigena D. Don ex Tilloch & Taylor, and C. paxii Kük.) to 1C = 1.64 pg ( C. cuspidate Bertol.) (data from the Plant DNA C-values Database; release 7.1). The number of species covered with this data corresponds to approximately 14% of reported Carex species worldwide.
In the present study, the genome sizes of 43 Carex species and infraspecific taxa distributed in the Korean peninsula were determined using flow cytometry. Although 180 Carex species and infraspecific taxa have been reported in the Korean peninsula ( Park, 2007), intensive studies of their genome sizes have not yet been performed. To understand genome size variation in each subgroup of Carex, we performed a combined analysis for our data with previously reported Carex genome size data deposited in the Plant DNA C-values Database.
Material and Methods
Plant material
Forty-three species and infraspecific taxa of Korean Carex were collected in the field and transplanted in the Sungshin Women’s University, Seoul, Korea. Voucher specimens were deposited in the herbarium of the Sungshin Women's University (SWU) ( Table 1). Subgeneric and sectional classification of these taxa followed Egorova (1999) and scientific names followed a recent manual for grasses and sedges in Korea ( Cho et al., 2016). Plant standards for flow cytometry have been proposed in Doležel et al. (2007). We requested seeds of Raphanus sativus L. ‘Saxa’ (2C = 1.11 pg), Solanum lycopersicum L. ‘Stupické polní rané’ (2C = 1.96 pg), and Glycine max Merr. ‘Polanka’ (2C = 2.50 pg) from Dr. Jaroslav Doležel (Laboratory of Molecular Cytogenetics and Cytometry, Institute of Experimental Botany, Olomouc, Czech Republic) and used them for the analyses. Seeds were germinated and grown in the greenhouse at Sungshin Women’s University and fully grown leaves were used for flow cytometry.
Sample preparation and flow cytometry
The genome size of each plant was estimated using flow cytometry as described in Doležel et al. (2007). Fresh leaves from a standard plant and a sample for estimation (each ca. 0.5 cm 2) were co-chopped using a razor blade in a Petri dish with Otto I buffer (0.1 M citric acid plus 0.5% Tween 20) ( Galbraith et al., 1983; Otto, 1990). The nuclear suspension was filtered through a 30 um nylon filter (CellTrics, Partec GmbH, Görlitz, Germany). The filtered suspension was added to Otto II buffer (0.4 M Na 2HPO 4 · 12H 2O) (Doležel and Göhde, 1995) containing 50 mg/mL propidium iodide (PI; Sigma-Aldrich, Sigma-Aldrich, St. Louis, MO, USA) and 50 mg/mL RNase A (Thermo Fisher Scientific Inc., Waltham, MA, USA). Samples were analyzed on an SH800S Cell Sorter (Sony Biotechnology Inc., San Jose, CA, USA). Each analysis was repeated at least three times on different days, except for C. dispalata, C. mitrata var. mitrata, C. pediformis var. pedunculata, C. kobomugi, and C. biwensis, which were repeated twice. At least 8,000 particles were measured in each sample, and the mean number of channels and coefficient of variation of the DNA peak (CV% = Standard deviation of the peak/mean channel number of the peak × 100) were evaluated. The final data only includes samples with a CV of less than 5% as suggested by Loureiro et al. (2007). The 2C-value was calculated based on the relative counts between G1 (growth 1 stage on the cell division) peaks from a standard plant and from a sample for the estimation. Finally, the genome size (bp) was calculated by the equation suggested in Doležel et al. (2007): 1C (pg) DNA = 0.978 × 10 9 bp.
Statistical analysis for combined genome size data
To address genome size statistics in each subgroup in Carex, we added our new data to the previously reported genome size data deposited in the Plant DNA C-value Database. When a species has multiple accessions, we used an average value for statistical analyses to minimize data bias. The minimum, maximum, average, and standard deviations were calculated in each subgenus and section based on Egorova’s classification system in Carex ( Egorova, 1999).
Results and Discussion
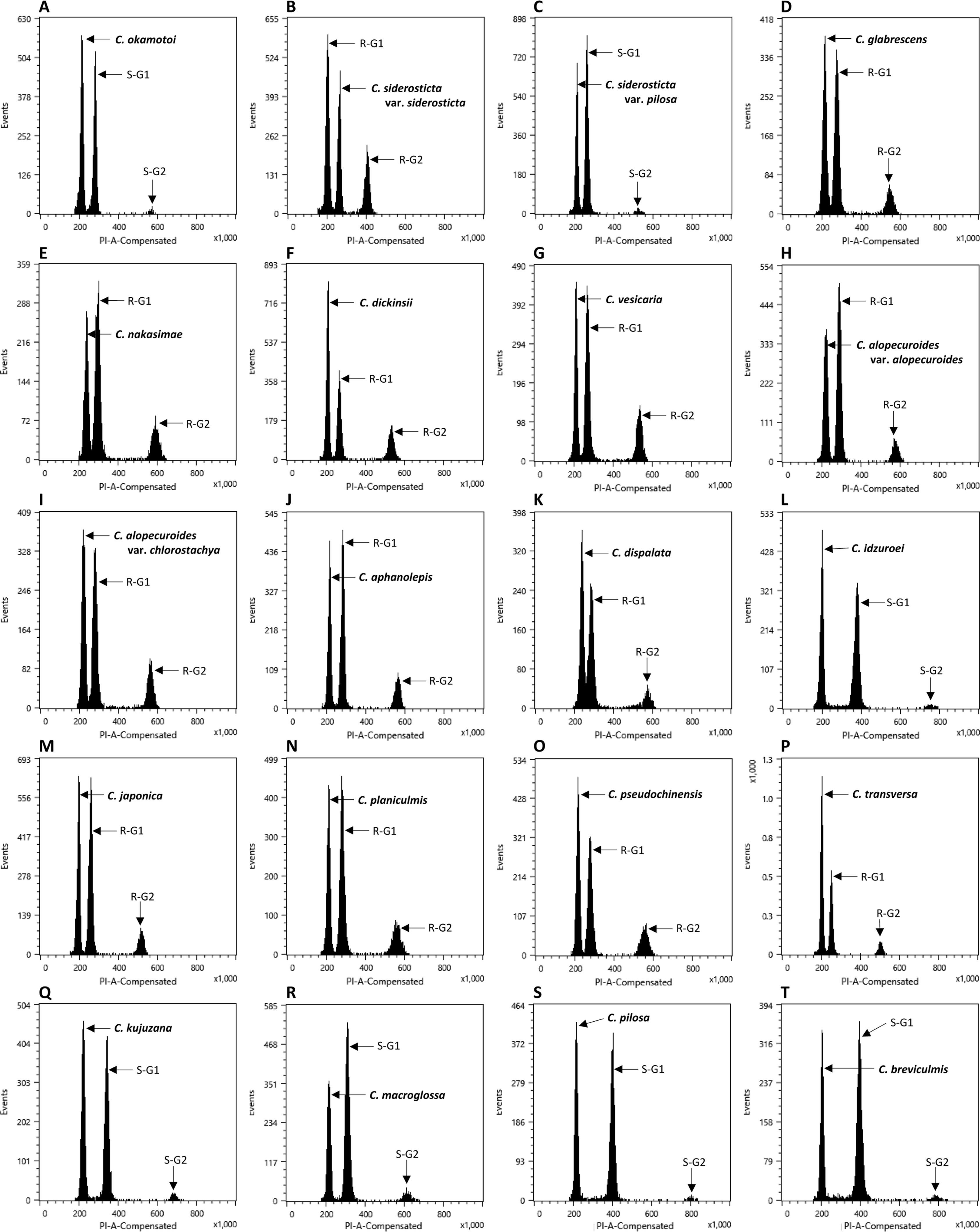
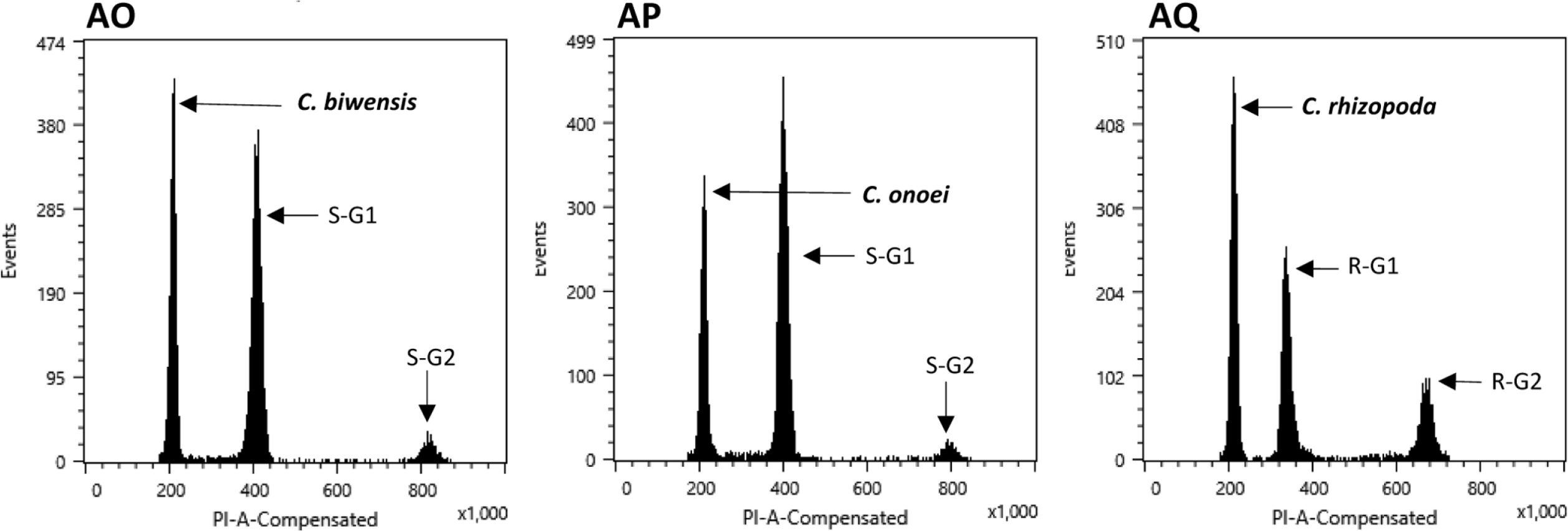
The flow cytometry results showed well-resolved histograms ( Fig. 1) with 2C peaks from a target sample and a plant standard having 2.20–4.69% CV, which is within acceptable infraspecific variation limits suggested by Doležel and Bartos (2005). We now report for the first time, genome size data from 43 species and infraspecific taxa of Carex distributed in the Korean peninsula ( Fig. 1, Table 1). Our results provide a 10.7% increase in data at the species level, against the existing genome size data for Carex (292 species and infraspecific taxa; 276 species; data from the Plant DNA C-Value Database). From our analyses of Korean Carex ( Table 1), 2C-values range from 0.55 pg ( C. neurocarpa Maxim.) to 2.25 pg ( C. pediformis var. pedunculata Maxim.), thus making 1C-values that range from 0.28 pg (0.27 Gbp) to 1.13 pg (1.10 Gbp). Seven taxa in our estimation overlapped with data in the Plant DNA C-values Database, and their 1C-values were slightly different ( Table 1). First of all, we cannot exclude the possibility of sample misidentification. However, the major contribution to differences result from different methodologies related to measurement technique, reference standard, staining dye, chemical interference, etc. (as discussed in Lipnerová et al., 2013; Wang et al., 2016; Yan et al., 2016). C. siderosticta Hance var. siderosticta, C. siderosticta var. pilosa H. Lév. ex T. Koyama, C. paxii Kük., and C. kobomugi Ohwi were estimated by Feulgen microdensitometry in the previous estimations, while we used flow cytometry with PI ( Nishikawa et al., 1984; Leitch et al., 2019). Although C. vesicaria L., C. pilosa Scop., and C. lanceolata Boott were estimated using S. lycopersicum L. ‘Stupické polní rané’ (1C = 0.87 pg), C. acutiformis Ehrh. (1C = 0.41 pg), and Oryza sativa subsp. japonica ‘Nipponbare’ (1C = 0.40 pg) as standard plants in the previous study ( Lipnerová et al., 2013), we used R. sativus L. ‘Saxa’ (2C = 1.11 pg), S. lycopersicum L. ‘Stupické polní rané’ (2C = 1.96 pg), and G. max Merr. ‘Polanka’ (2C = 2.50 pg). These plant standards were calibrated using human male leukocytes (2C = 7.0 pg) which was overestimated compared to modern sequencing data ( Doležel and Greilhuber, 2010; Lipnerová et al., 2013). The Plant DNA C-Value Database with our data included, contains a total of 372 Carex accessions. We identified the taxonomic position of these accessions based on Egorova’s classification system of Carex ( Egorova, 1999) and performed statistical analyses in each subgroup of the Carex. We excluded accessions showing unclear sectional membership, which left data from 248 Carex species remaining ( Table 2). The final data set reveals that the average genome size of Carex is 0.47 ± 0.19 pg (1C) ( Table 2). C. cuspidate Bertol. has the largest genome (1C = 1.64 pg) and four species equally ( C. brownii Tuck., C. kobomugi Ohwi, C. nubigena D. Don ex Tilloch & Taylor, and C. paxii Kük.) have the smallest genome (1C = 0.20 pg). The large genomes are frequently found in the subgen. Carex compare to other subgenera ( Fig. 2), especially in sect. Aulocystis, sect. Digitatae, sect. Glaucae, sect. Paniceae, and sect. Siderostictae. However, genome size variations in these sections were also relatively high. The sect. Secalinae of the subgen. Carex has the lowest mean value in the sectional level (1C = 0.25) ( Table 2). Also, the mean value of subg. Vignea is relatively small (1C = 0.37) ( Fig. 2, Table 2) and is consistent with the result of Lipnerová et al. (2013). We provided the first intensive genome size information of Carex distributed in Korea and our data updates the overall current understanding of the genome size in Carex. This data will form the basis for understanding the phylogeny and evolution of Carex as well as genetic studies on Korean endemic species. In addition, our data will be useful information for future intensive genome sequencing projects in Carex.
ACKNOWLEDGMENTS
This work was supported by the Sungshin University Research Grant of 2017. The authors thank Dr. Jaroslav Doležel for providing seeds of standard plants for flow cytometry.
Fig. 2.
Range of genome size variations (1C value) in each subgenus ( A) and section ( B) in Carex. Boxes indicate the range of standard deviations and ends of bars indicate the maximum and the minimum values. The number of species included in each subgenus and section is in Table 2. 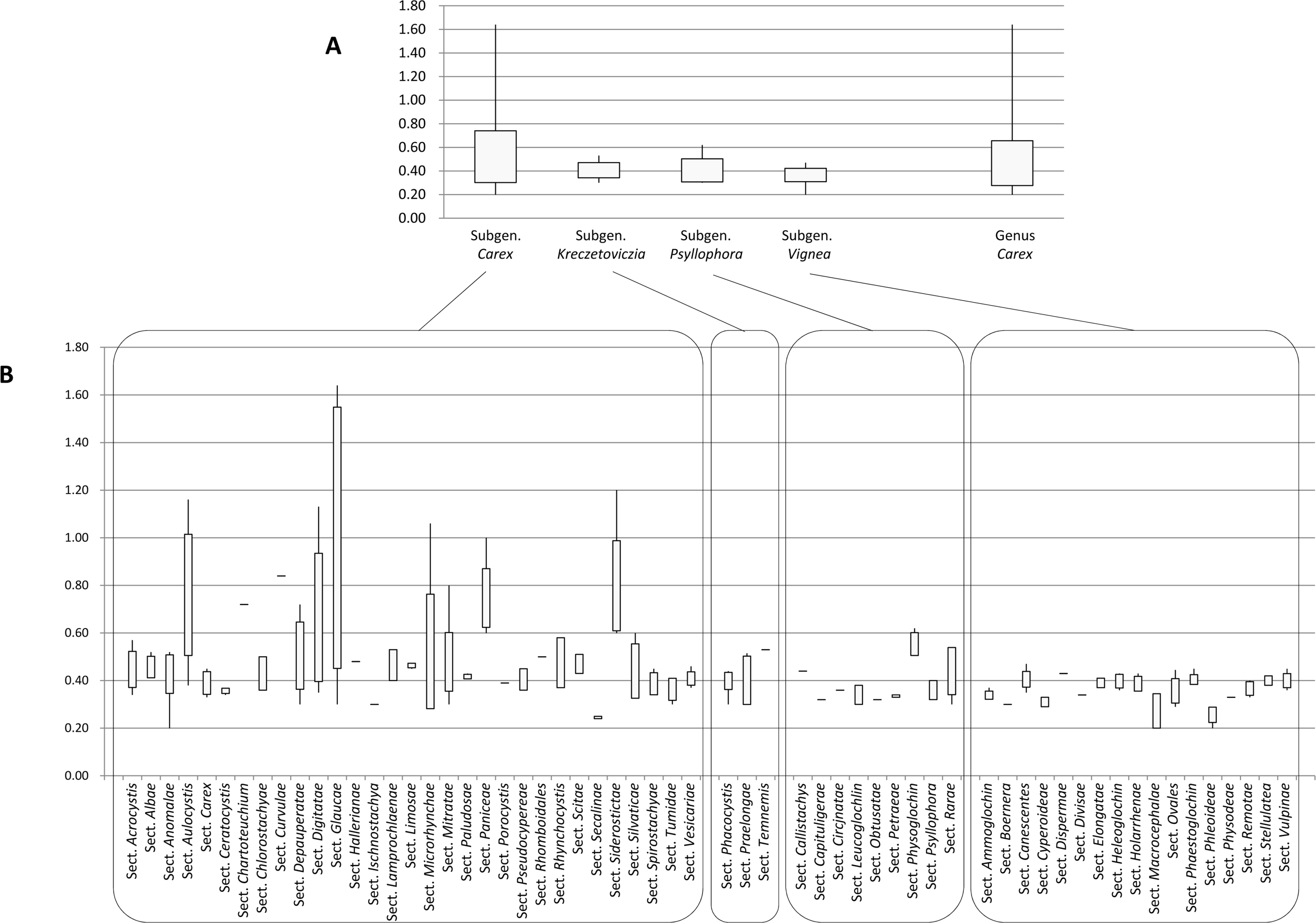
Table 1.
Genome size and voucher information of Korean Carex included in this study.
|
Subgenus |
Section |
Taxon (Korean name)a
|
1C-value ± SDb (pg) |
CV (%) ± SD |
Genome size (Gbp) |
Standard plantc
|
Previous reported 1C (pg) |
Voucher |
|
Carex
|
Siderostictae
|
C. okamotoi Ohwi (지리대사초) |
0.74 ± 0.02 |
3.73 ± 0.46 |
0.72 |
S |
|
B. Lee 2017-008, B.
Lee 2017-029
|
|
C. siderosticta Hance var. siderosticta (대사초) |
0.77 ± 0.05 |
3.66 ± 1.08 |
0.75 |
R |
1.2 (Nishikawa et al., 1984) |
B. Lee 2017-030, B.
Lee 2017-047, B.
Lee 2019-080
|
|
C. siderosticta var. pilosa H. Lév. ex T. Koyama (털대사초) |
0.78 ± 0.02 |
3.26 ± 0.58 |
0.76 |
S |
0.6 (Hanson et al., unpublished) |
B. Lee 2017-006, B.
Lee 2019-081
|
|
Carex
|
C. glabrescens (Kük.) Ohwi (곱슬사초) |
0.44 ± 0.01 |
4.26 ± 0.55 |
0.43 |
R |
|
B. Lee 2019-069
|
|
C. nakasimae Ohwi (화산사초) |
0.45 ± 0.01 |
3.85 ± 0.17 |
0.44 |
R |
|
B. Lee 2019-068
|
|
Vesicariae
|
C. dickinsii Franch. & Sav. (도깨비사초) |
0.46 ± 0.03 |
3.33 ± 0.28 |
0.45 |
R |
|
S.Kim2019-116, S.Kim2019-117
|
|
C. vesicaria L. (새방울사초) |
0.44 ± 0.02 |
3.58 ± 0.11 |
0.43 |
R |
0.4 (Lipnerová et al., 2013) |
B. Lee 2019-066
|
|
Anomalae
|
C. alopecuroides D. Don var. alopecuroides (가는흰사초) |
0.42 ± 0.02 |
3.91 ± 0.51 |
0.41 |
R |
|
B. Lee 2017-002
|
|
C. alopecuroides var. chlorostachya C. B. Clarke (흰사초) |
0.47 ± 0.03 |
3.56 ± 0.45 |
0.46 |
R |
|
B. Lee 2017-026, B.
Lee 2019-060
|
|
C. aphanolepis Franch. & Sav. (골사초) |
0.42 ± 0.02 |
3.62 ± 0.22 |
0.41 |
R |
|
B. Lee 2019-064
|
|
C. dispalata Boott ex A. Gray (삿갓사초) |
0.47 ± 0.00 |
3.41 ± 0.07 |
0.47 |
R |
|
B. Lee 2019-072, B.
Lee 2014-077
|
|
C. idzuroei Franch. & Sav. (좀도깨비사초) |
0.52 ± 0.00 |
3.91 ± 0.27 |
0.51 |
S |
|
B. Lee 2019-071
|
|
C. japonica Thunb. (개찌버리사초) |
0.42 ± 0.02 |
3.86 ± 0.14 |
0.41 |
R |
|
B. Lee 2017-028
|
|
C. planiculmis Kom. (그늘흰사초) |
0.45 ± 0.03 |
3.93 ± 0.11 |
0.44 |
R |
|
B. Lee 2017-033, B.
Lee 2017-045
|
|
C. pseudochinensis H. Lév. & Vaniot (햇사초) |
0.45 ± 0.01 |
3.50 ± 0.33 |
0.44 |
R |
|
B. Lee 2019-065
|
|
C. transversa Boott (화살사초) |
0.45 ± 0.02 |
3.46 ± 0.54 |
0.44 |
R |
|
B. Lee 2019-055, B.
Lee 2019-058
|
|
Depauperatae
|
C. kujuzana Ohwi (장성사초) |
0.64 ± 0.00 |
4.07 ± 0.18 |
0.62 |
S |
|
B. Lee 2017-016
|
|
C. macroglossa Franch. & Sav. (애기염주사초) |
0.69 ± 0.00 |
4.02 ± 0.25 |
0.67 |
S |
|
B. Lee 2019-054
|
|
C. pilosa Scop. (털사초) |
0.53 ± 0.00 |
3.83 ± 0.24 |
0.52 |
S |
0.48 (Lipnerová et al., 2013) |
B. Lee 2017-044
|
|
Mitratae
|
C. breviculmis R. Br. (청사초) |
0.51 ± 0.00 |
3.57 ± 0.54 |
0.49 |
S |
|
B. Lee 2019-082
|
|
C. genkaiensis Ohwi (목포사초) |
0.60 ± 0.01 |
3.32 ± 0.44 |
0.59 |
S |
|
B. Lee 2019-052
|
|
C. kamagariensis K. Okamoto (큰청사초) |
0.48 ± 0.01 |
3.47 ± 0.21 |
0.47 |
R |
|
B. Lee 2017-020
|
|
C. meridiana Akiyama (갯바위청사초) |
0.52 ± 0.01 |
3.47 ± 0.67 |
0.51 |
S |
|
B. Lee 2019-059
|
|
C. mitrata Franch. var. mitrata (겨사초) |
0.51 ± 0.01 |
3.40 ± 0.16 |
0.49 |
S |
|
B. Lee 2017-022
|
|
C. mitrata var. aristata Ohwi (까락겨사초) |
0.49 ± 0.00 |
3.12 ± 0.22 |
0.48 |
R |
|
B. Lee 2019-053
|
|
C. polyschoena H. Lev. & Vaniot (가지청사초) |
0.50 ± 0.01 |
3.90 ± 0.85 |
0.48 |
S |
|
B. Lee 2017-011
|
|
C. sabynensis Less. ex Kunth (실청사초) |
0.59 ± 0.00 |
3.42 ± 0.82 |
0.57 |
S |
|
B. Lee 2017-034
|
|
C. subebracteata (Kuk.) Ohwi (부리실청사초) |
0.57 ± 0.01 |
3.86 ± 0.93 |
0.56 |
S |
|
B. Lee 2017-048
|
|
C. tristachya Thunb. (반들사초) |
0.58 ± 0.00 |
3.18 ± 0.44 |
0.56 |
S |
|
B. Lee 2019-061
|
|
Digitatae
|
C. lanceolata Boott (그늘사초) |
1.10 ± 0.01 |
2.38 ± 0.42 |
1.07 |
G |
0.87 (Lipnerová et al., 2013) |
B. Lee 2017-004
|
|
C. macrandrolepis H. Lév. & Vaniot (청피사초) |
0.54 ± 0.01 |
3.36 ± 0.31 |
0.52 |
S |
|
B. Lee 2019-057
|
|
C. pediformis var. pedunculata Maxim. (왕그늘사초) |
1.13 ± 0.00 |
2.20 ± 0.21 |
1.10 |
G |
|
S. Kim 2019-018
|
|
Acrocystis
|
C. fusanensis Ohwi (부산사초) |
0.37 ± 0.00 |
3.18 ± 0.45 |
0.36 |
R |
|
B. Lee 2017-007
|
|
Microrhynchae
|
C. peiktusani Kom. (백두사초) |
1.06 ± 0.00 |
2.27 ± 0.24 |
1.04 |
G |
|
B. Lee 2017-046
|
|
Kreczetoviczia
|
Praelongae
|
C. dimorpholepis Steud. (이삭사초) |
0.52 ± 0.01 |
3.52 ± 0.71 |
0.50 |
S |
|
B. Lee 2014-079
|
|
C. phacota Spreng. (비늘사초) |
0.49 ± 0.00 |
3.44 ± 0.18 |
0.48 |
S |
|
B. Lee 2017-024
|
|
Vignea
|
Phleoideae
|
C. neurocarpa Maxim. (괭이사초) |
0.28 ± 0.00 |
4.06 ± 0.68 |
0.27 |
R |
|
S. Kim 2019-060
|
|
C. paxii Kük. (대구사초) |
0.29 ± 0.01 |
4.69 ± 0.33 |
0.28 |
R |
0.2 (Nishikawa et al., 1984) |
B. Lee 2019-073
|
|
Macrocephalae
|
C. kobomugi Ohwi (통보리사초) |
0.35 ± 0.00 |
3.38 ± 0.17 |
0.34 |
R |
0.2 (Nishikawa et al., 1984) |
B. Lee 2019-083
|
|
Ovales
|
C. maackii Maxim. (타래사초) |
0.45 ± 0.01 |
3.02 ± 0.39 |
0.44 |
R |
|
B. Lee 2019-067
|
|
Psyllophora
|
Rarae
|
C. biwensis Franch. (솔잎사초) |
0.51 ± 0.00 |
3.27 ± 0.21 |
0.49 |
S |
|
B. Lee 2017-027
|
|
C. onoei Franch. & Sav. (바늘사초) |
0.52 ± 0.01 |
3.17 ± 0.49 |
0.50 |
S |
|
B. Lee 2017-051
|
|
Circinatae
|
C. rhizopoda Maxim. (뿌리대사초) |
0.36 ± 0.02 |
3.57 ± 0.39 |
0.35 |
R |
|
B. Lee 2019-062
|
Table 2.
Genome size of subgroups in Carex reported to date including this study.
|
Subgenus |
Section |
No. of species |
Min. |
Max. |
Mean |
SD |
|
Carex
|
|
151
|
0.20
|
1.64
|
0.52
|
0.22
|
|
Acrocystis
|
8 |
0.34 |
0.57 |
0.45 |
0.08 |
|
Albae
|
3 |
0.42 |
0.52 |
0.46 |
0.04 |
|
Anomalae
|
10 |
0.20 |
0.52 |
0.43 |
0.08 |
|
Aulocystis
|
8 |
0.38 |
1.16 |
0.76 |
0.25 |
|
Carex
|
5 |
0.33 |
0.45 |
0.39 |
0.05 |
|
Ceratocystis
|
6 |
0.34 |
0.37 |
0.36 |
0.01 |
|
Chartoteuchium
|
1 |
0.72 |
0.72 |
0.72 |
0.00 |
|
Chlorostachyae
|
2 |
0.36 |
0.50 |
0.43 |
0.07 |
|
Curvulae
|
1 |
0.84 |
0.84 |
0.84 |
0.00 |
|
Depauperatae
|
9 |
0.30 |
0.72 |
0.50 |
0.14 |
|
Digitatae
|
13 |
0.35 |
1.13 |
0.67 |
0.27 |
|
Glaucae
|
3 |
0.30 |
1.64 |
1.00 |
0.55 |
|
Hallerianae
|
1 |
0.48 |
0.48 |
0.48 |
0.00 |
|
Ischnostachya
|
1 |
0.30 |
0.30 |
0.30 |
0.00 |
|
Lamprochlaenae
|
2 |
0.40 |
0.53 |
0.47 |
0.07 |
|
Limosae
|
3 |
0.45 |
0.47 |
0.46 |
0.01 |
|
Microrhynchae
|
9 |
0.30 |
1.06 |
0.52 |
0.24 |
|
Mitratae
|
21 |
0.30 |
0.80 |
0.48 |
0.12 |
|
Paludosae
|
3 |
0.41 |
0.43 |
0.42 |
0.01 |
|
Paniceae
|
6 |
0.60 |
1.00 |
0.75 |
0.12 |
|
Porocystis
|
1 |
0.39 |
0.39 |
0.39 |
0.00 |
|
Pseudocypereae
|
2 |
0.36 |
0.45 |
0.41 |
0.04 |
|
Rhomboidales
|
4 |
0.50 |
0.50 |
0.50 |
0.00 |
|
Rhynchocystis
|
2 |
0.37 |
0.58 |
0.48 |
0.11 |
|
Scitae
|
2 |
0.43 |
0.51 |
0.47 |
0.04 |
|
Secalinae
|
2 |
0.24 |
0.25 |
0.25 |
0.01 |
|
Siderostictae
|
6 |
0.60 |
1.20 |
0.80 |
0.19 |
|
Silvaticae
|
3 |
0.34 |
0.60 |
0.44 |
0.11 |
|
Spirostachyae
|
3 |
0.34 |
0.45 |
0.39 |
0.05 |
|
Tumidae
|
3 |
0.30 |
0.41 |
0.36 |
0.05 |
|
Vesicariae
|
8 |
0.37 |
0.46 |
0.41 |
0.03 |
|
Kreczetoviczia
|
|
18
|
0.30
|
0.53
|
0.41
|
0.06
|
|
Phacocystis
|
13 |
0.30 |
0.44 |
0.40 |
0.04 |
|
Praelongae
|
4 |
0.30 |
0.52 |
0.40 |
0.10 |
|
Temnemis
|
1 |
0.53 |
0.53 |
0.53 |
0.00 |
|
Psyllophora
|
|
16
|
0.30
|
0.62
|
0.41
|
0.10
|
|
Callistachys
|
1 |
0.44 |
0.44 |
0.44 |
0.00 |
|
Capituligerae
|
1 |
0.32 |
0.32 |
0.32 |
0.00 |
|
Circjnatae
|
1 |
0.36 |
0.36 |
0.36 |
0.00 |
|
Leucoglochlin
|
2 |
0.30 |
0.38 |
0.34 |
0.04 |
|
obtusatae
|
1 |
0.32 |
0.32 |
0.32 |
0.00 |
|
Petraeae
|
2 |
0.33 |
0.34 |
0.34 |
0.01 |
|
Physoglochin
|
3 |
0.51 |
0.62 |
0.55 |
0.05 |
|
Psyllophora
|
2 |
0.32 |
0.40 |
0.36 |
0.04 |
|
Rarae
|
3 |
0.30 |
0.52 |
0.44 |
0.10 |
|
Vignea
|
|
63
|
0.20
|
0.47
|
0.37
|
0.06
|
|
Ammoglochin
|
7 |
0.32 |
0.37 |
0.34 |
0.02 |
|
Boernera
|
1 |
0.30 |
0.30 |
0.30 |
0.00 |
|
Canescentes
|
11 |
0.35 |
0.47 |
0.41 |
0.03 |
|
Cyperoideae
|
2 |
0.29 |
0.33 |
0.31 |
0.02 |
|
Dispermae
|
1 |
0.43 |
0.43 |
0.43 |
0.00 |
|
Divisae
|
2 |
0.34 |
0.34 |
0.34 |
0.00 |
|
Elongatae
|
2 |
0.37 |
0.41 |
0.39 |
0.02 |
|
Heleoglochin
|
4 |
0.36 |
0.43 |
0.40 |
0.03 |
|
Holarrhenae
|
3 |
0.36 |
0.43 |
0.39 |
0.03 |
|
Ovales
|
2 |
0.20 |
0.35 |
0.27 |
0.07 |
|
Phaestoglochin
|
5 |
0.29 |
0.45 |
0.36 |
0.05 |
|
Phleoideae
|
7 |
0.39 |
0.45 |
0.40 |
0.02 |
|
Physodeae
|
5 |
0.20 |
0.29 |
0.26 |
0.03 |
|
Remotae
|
1 |
0.33 |
0.33 |
0.33 |
0.00 |
|
Stellulatea
|
3 |
0.33 |
0.40 |
0.37 |
0.03 |
|
Vulpinae
|
2 |
0.38 |
0.42 |
0.40 |
0.02 |
|
Total
|
|
248
|
0.20
|
1.64
|
0.47
|
0.19
|
Literature Cited
Bennett, MD and Leitch, IJ. 2011. Nuclear DNA amounts in angiosperms: targets, trends and tomorrow. Annals of Botany 107: 467-590.   Bennett, MD and Smith, JB. 1976. Nuclear DNA amounts in angiosperms. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 274: 227-274.
Cho, Y. Kim, J-W and Park, SH. 2016. Grasses and Sedges in South Korea. Geobook Co., Seoul. 527 (in Korean)..
Chung, K-S. Hipp, AL and Roalson, EH. 2012. Chromosome number evolves independently of genome size in a clade with nonlocalized centromeres ( Carex: Cyperaceae). Evolution 66: 2708-2722.  Chung, K-S. Hoshino, T. Masaki, T. Yang, JC and Im, H-T. 2016. Cytological studies on seven species of Korean Carex (Cyperaceae). Cytologia 81: 143-147.  Doležel, J and Bartoš, J. 2005. Plant DNA flow cytometry and estimation of nuclear genome size. Annals of Botany 95: 99-110.   Doležel, J and Göhde, W. 1995. Sex determination in dioecious plants Melandrium album and M. rubrum using high-resolution flow cytometry. Cytometry 19: 103-106.  Doležel, J and Greilhuber, J. 2010. Nuclear genome size: are we getting closer? Cytometry Part A 77A: 635-642.  Doležel, J. Greilhuber, J and Suda, J. 2007. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols 2: 2233-2244.   Egorova, TV. 1999. The Sedges (Carex L.) of Russia and Adjacent States (within the Limits of the former USSR). St-Petersburg State Chemical-Pharmaceutical Academy, St-Petersburg and Missouri Botanical Garden Press, St. Louis, MO. 772 pp.
Escudero, M. Hipp, AL. Waterway, MJ and Valente, LM. 2012. Diversification rates and chromosome evolution in the most diverse angiosperm genus of the temperate zone ( Carex, Cyperaceae). Molecular Phylogenetics and Evolution 63: 650-655.  Fleischmann, A. Michael, TP. Rivadavia, F. Sousa, A. Wang, W. Temsch, EM. Greilhuber, J. Müller, KF and Heubl, G. 2014. Evolution of genome size and chromosome number in the carnivorous plant genus Genlisea (Lentibulariaceae), with a new estimate of the minimum genome size in angiosperms. Annals of Botany 114: 1651-1663.   Galbraith, DW. Harkins, KR. Maddox, JM. Ayres, NM. Sharma, DP and Firoozabady, E. 1983. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220: 1049-1051.  Global Carex Group. 2015. Making Carex monophyletic (Cyperaceae, tribe Cariceae): a new broader circumscription. Botanical Journal of the Linnean Society 179: 1-42.
Grover, CE and Wendel, JF. 2010. Recent insights into mechanisms of genome size change in plants. Journal of Botany 2010: 382732.   Hipp, AL. Escudero, M and Chung, K-S. 2013. Holocentric chromosomes. Brenner’s Encyclopedia of Genetics. 2nd ed.. Maloy, S. Hughes, K (eds.), Elsevier Inc, New York. 499-501.  Hipp, AL. Rothrock, PE and Roalson, EH. 2009. The evolution of chromosome arrangements in Carex (Cyperaceae). The Botanical Review 75: 96-109.   Hipp, AL. Rothrock, PE. Whitkus, R and Weber, JA. 2010. Chromosomes tell half of the story: the correlation between karyotype rearrangements and genetic diversity in sedges, a group with holocentric chromosomes. Molecular Ecology 19: 3124-3138.  Kejnovsky, E. Hawkins, JS and Feschotte, C. 2012. Plant transposable elements: biology and evolution. Plant Genome Diversity. 1: Wendel, J. Greilhuber, J. Dolezel, J. Leitch, I (eds.), Springer, Vienna. 17-34.  Leitch, IJ. Johnston, E. Pellicer, J. Hidalgo, O and Bennett, MD. 2019. Angiosperm DNA C-values database (release 9.0, April 2019). Retrieved Jul. 7, 2019. available from https://cvalues.science.kew.org/.. Leitch, AR and Leitch, IJ. 2012. Ecological and genetic factors linked to contrasting genome dynamics in seed plants. New Phytologist 194: 629-646.  Lipnerová, I. Bureš, P. Horová, L and Šmarda, P. 2013. Evolution of genome size in Carex (Cyperaceae) in relation to chromosome number and genomic base composition. Annals of Botany 111: 79-94.   Loureiro, J. Suda, J. Doležel, J and Santos, C. 2007. FLOWER: a plant DNA flow cytometry database. Flow Cytometry with Plant Cells: Analysis of Genes, Chromosomes and Genomes. Doležel, J. Greilhuber, J. Suda, J (eds.), Wiley-VCH, Weinheim. 423-438.  Nishikawa, K. Furuta, Y and Ishitobi, K. 1984. Chromosomal evolution in genus Carex as viewed from nuclear DNA content, with special reference to its aneuploidy. The Japanese Journal of Genetics 59: 465-472.  Otto, F. 1990. DAPI Staining of fixed cells for high-resolution flow cytometly of nuclear DNA. Methods in Cell Biology 33: 105-110.  Park, C-W. 2007. The Genera of Vascular Plants of Korea. Flora of Korea Editorial Committee. Academy Publishing Co., Seoul. 1482 (in Korean).
Pellicer, J. Fay, MF and Leitch, IJ. 2010. The largest eukaryotic genome of them all? Botanical Journal of the Linnean Society 164: 10-15.   Tanaka, N. 1949. Chromosome studies in the genus Carex, with special reference to aneuploidy and polyploidy. Cytologia 15: 15-29.  Wang, N. McAllister, HA. Bartlett, PR and Buggs, RJA. 2016. Molecular phylogeny and genome size evolution of the genus Betula (Betulaceae). Annals of Botany 117: 1023-1035.   Yan, H. Martin, SL. Bekele, WA. Latta, RG. Diederichsen, A. Peng, Y and Tinker, NA. 2016. Genome size variation in the genus Avena. Genome 59: 209-220.  Zonneveld, BJM. Leitch, IJ and Bennett, MD. 2005. First nuclear DNA amounts in more than 300 angiosperms. Annals of Botany 96: 229-244.  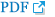
|
|