The allopolyploid origin of Euphorbia stevenii and E. boöphthona (Euphorbiaceae)
Article information
Abstract
To elucidate the ancestry of the allopolyploids E. stevenii and E. boöphthona, I examined eleven isozyme loci and 24 morphological characters from 28 populations representing five related Euphorbia species from Australia. According to an analysis of genetic and morphological data, three diploid species differentiated recently, but two independent polyploid species are estimated to have differentiated a relatively long time ago. Fixed heterozygosity for most isozymes in E. stevenii and E. boöphthona strongly suggests that these two species are allopolyploids rather than autopolyploids. The isozyme profiles of E. stevenii indicate that it is an allopolyploid that evolved from interspecific hybridization between the diploid E. tannensis and unidentified or extinct tetraploid species. In addition, isozyme patterns strongly suggest that E. stevenii was one of the ancestors of E. boöphthona. However, E. boöphthona showed a large number of fixed alleles that were not detected in any other Australian Eremophyton species. The most likely hypothesis for the origin of E. boöphthona is that it was formed by hybridization and chromosomal doubling between an extinct diploid species and the hexaploid E. stevenii.
INTRODUCTION
Polyploidy has been considered as a significant aspect of plant evolution, and contributed greatly to the diversity of vascular plants (Grant, 1981; Werth, 1989; Arnold, 1997). Among Euphorbiaceae, the great diversity of chromosome numbers and size has significantly contributed to the species diversity in the genus Euphorbia L. (Perry, 1943; Hans, 1973; Urbatsch et al., 1975). In fact, Hans (1973) reported that about 40% of the Euphorbia species were polyploids. Although relatively many cases of the polyploid origin have been hypothesized in Euphorbia, the origin and parentage of polyploid species were not well documented genetically.
The Australian species of Euphorbia section Eremophyton Boiss. consist of five endemic species, and they are regarded as a natural group with same basic chromosome number (x = 7) (Hassall, 1976, 1977). Of the five species in this section, E. tannensis Spreng., the most widespread species (2n = 14), was traditionally treated as three different species (E. tannensis, E. eremophila A. Cunn., and E. finlaysonii J. M. Black), and later, Hassall (1977) treated them as two subspecies of E. tannensis. He also distinguished two varieties for subs. eremophila based on the gland shape. However, Forster (1992) only supported the recognition of two subspecies within the E. tannensis complex. Euphorbia parvicaruncula Hassall (2n = 14), usually occurring on hard duplex soils or desert loam in inland Australia, differs from other species by semi-succulent and swollen stem. The plains spurge, E. planiticola Hassall, probably a diploid, occurs on the open plains in New South Wales, and is morphologically confused with E. tannensis and E. stevenii Bailey (Hassall, 1977). Euphorbia boöphthona Gardner, the octoploid species (2n = 56), is widespread in the inland Australia, and is distinguished by the triangular and recurved fruits. Euphorbia stevenii, bottle-tree spurge, occurs in deeply cracking clay soils in recently flooded areas from central to south Australia. This species is hexaploid (2n = 42), but dodecaploid individuals were detected in the same population from Windorah, Queensland (Hassall, 1976).
Numerical study using morphological data was conducted within Australian Euphorbia species, and supported naturalness of the sect. Eremophyton (Hassall, 1976). However, the two subspecies of E. tannensis did not cluster together. The purpose of this study was to elucidate the origin of two polyploids from Australian Euphorbias using isozyme and morphological data.
MATERIALS AND METHODS
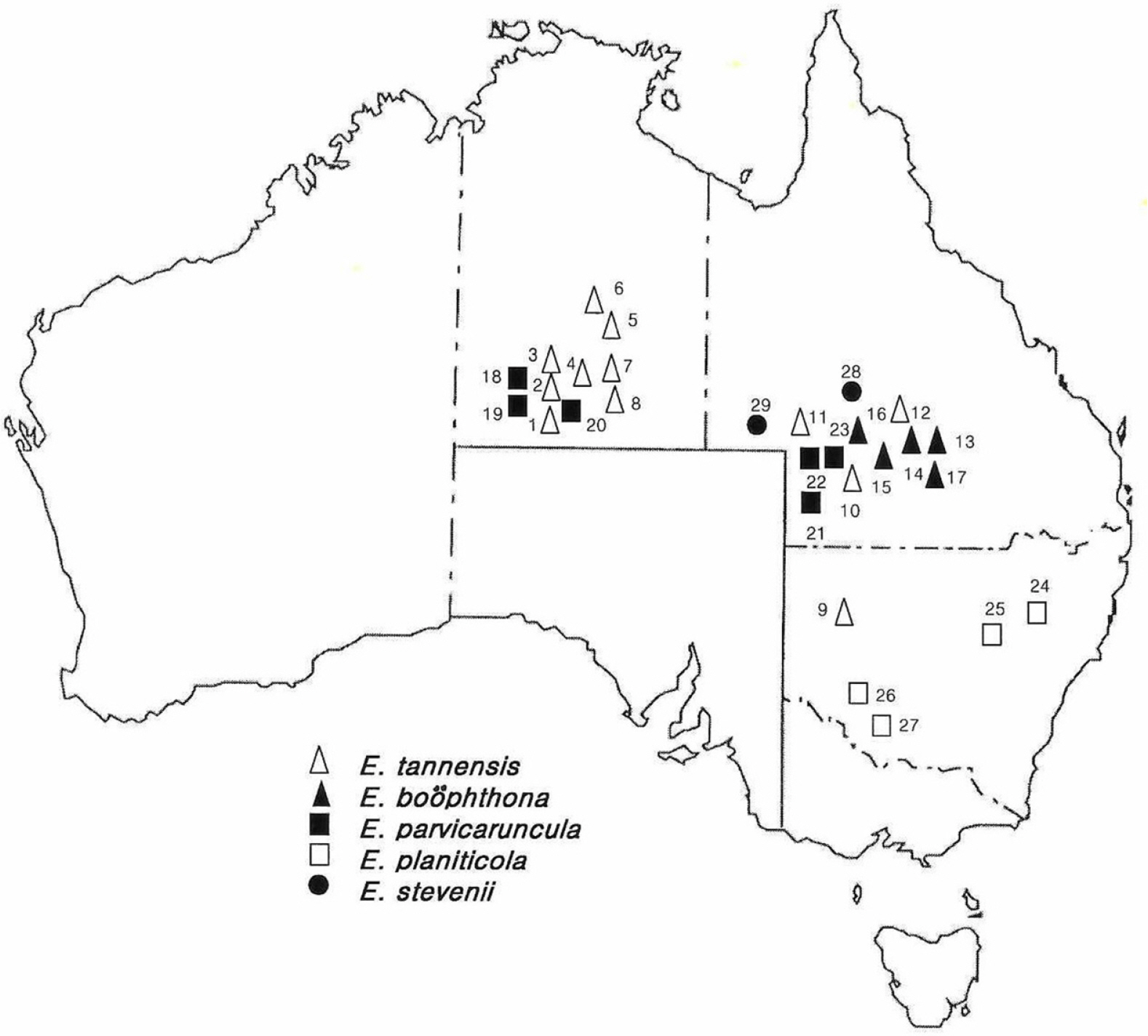
A total ca. 140 individuals from 28 populations of the five species were examined for morphological and electrophoretic analyses (Fig. 1, Table 1). Voucher specimens were deposited at the Herbarium of Kyung-Nam University (KNUH).

Collection data for 29 populations representing five species of Australian endemic Euphorbia sect. Eremophyton examined for isozyme and morphological studies.
Morphological analysis
Twenty-four characters (Table 2) were selected for morphological study among 28 populations of five Australian Euphorbia species. By use of field-collected dry specimens, the mean value of 10 measurements was obtained for each character per population. Clustering analysis was conducted using NTSYS-pc (Rohlf, 1992). Morphological data were standardized using default options of the STAND program, and the average taxonomic distance among populations was calculated by the SIMINT program. An unweighted pair-group method using arithmetic averages (UPGMA) phenogram was produced by the input of the distance matrix into the SAHN program of NTSYS-pc.
Isozyme analysis
Soluble enzymes were isolated from fresh leaf tissue of field-collected plants in an extracting buffer containing 0.1 M tris-HCl, pH 7.5, 1 mM EDTA (tetrasodium salt), 10 mM MgCl2, 10 mM KCl, 14 mM 2-mercaptoethanol, and 5–10 mg/mL solid polyvinylpolypyrrolidone (Gottlieb, 1981). Leaf extracts were centrifuged in 1.5 mL tubes and the supernatant was absorbed onto wicks of Whatman 17 MM chromatography paper. They were stored at −70oC until electrophoresed.
Ten enzymes were resolved on 11% starch gels with two buffer systems (Soltis et al., 1983). System I had an electrode buffer of 0.065 M L-histidine free base titrated to pH 6.5 with 0.007 M citric acid monohydrate and a gel buffer of a 1:3 dilution of the electrode buffer. System II consisted of an electrode buffer with 0.192 M boric acid titrated to pH 8.3 with LiOH, and a gel buffer a 1:9 dilution of the electrode buffer and 0.052 M Tris base and 0.008 M citric acid anhydrous, pH 8.3.
System I was used to resolve malate dehydrogenase (MDH), 6-phosphogluconate dehydrogenase (6PGD), isocitrate dehydrogenase (IDH), shikimic dehydrogenase (SKDH). System II was employed to resolve phosphoglucose isomerase (PGI), alcohol dehydrogenase (ADH), and malic enzyme (ME). Enzyme activity staining and agarose overlays mostly followed the protocols of Soltis et al. (1983). Loci and alleles were numbered sequentially and lettered alphabetically, beginning with the most anodal form.
BIOSYS-1 program (Swofford and Selander, 1981) was used to calculate Nei’s (1978) genetic identity among populations and to generate a UPGMA phenogram (Sneath and Sokal, 1973). The gene frequencies of polyploid species were based on the electrophoretic phenotype in the same way as for diploids. Therefore, the genetic identity coefficients among populations were calculated based on the same number of loci as diploid-diploid comparisons (Acquaah, 1992; Sun, 1996).
RESULTS
In the UPGMA phenogram depicted using morphological characters (Fig. 2), conspecific populations clustered together, except for the populations of E. tannensis. Twelve populations of E. tannensis were divided into two separate clusters. The three diploids, E. tannensis, E. parvicaruncula and E. planiticola, formed a cluster, and suggested a close relationship. However, two polyploid species formed different clusters.
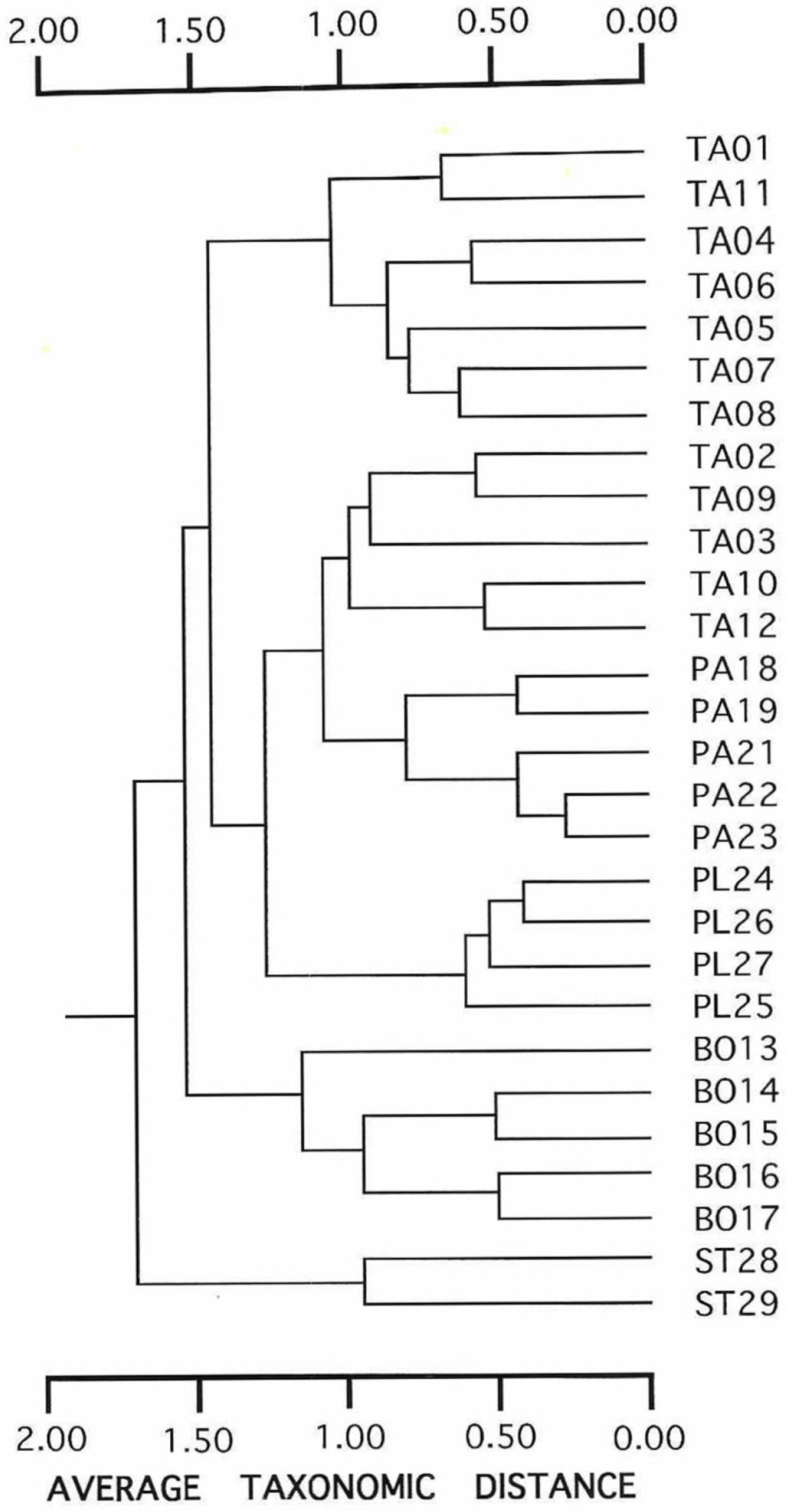
Unweighted pair group method with arithmetic mean phenogram based on average taxonomic distance coefficients using 24 morphological characters from 28 populations representing five species of Australian Euphorbia sect. Eremophyton. The cophenetic correlation is 0.763. Species abbreviations: BO, E. boöphthona; PA, E. parvicaruncula; PL, E. planiticola; TA, E. tannensis; ST, E. stevenii.
Eleven loci, coding for seven enzyme, were scored from 12 populations of five species of Euphorbia sect. Eremophyton. The number of isozymes showed clear differences between diploid and polyploid at all loci. The isozyme number of E. tannensis, E. parvicaruncula, and E. planiticola were similar to those reported for diploid species (Weeden and Wendel, 1989).
The UPGMA tree generated using genetic identity coefficients shows that the three diploid species, E. tannensis, E. parvicaruncula, and E. planiticola, formed a cluster, and they were clustered to E. stevenii at the next level (Fig. 3).
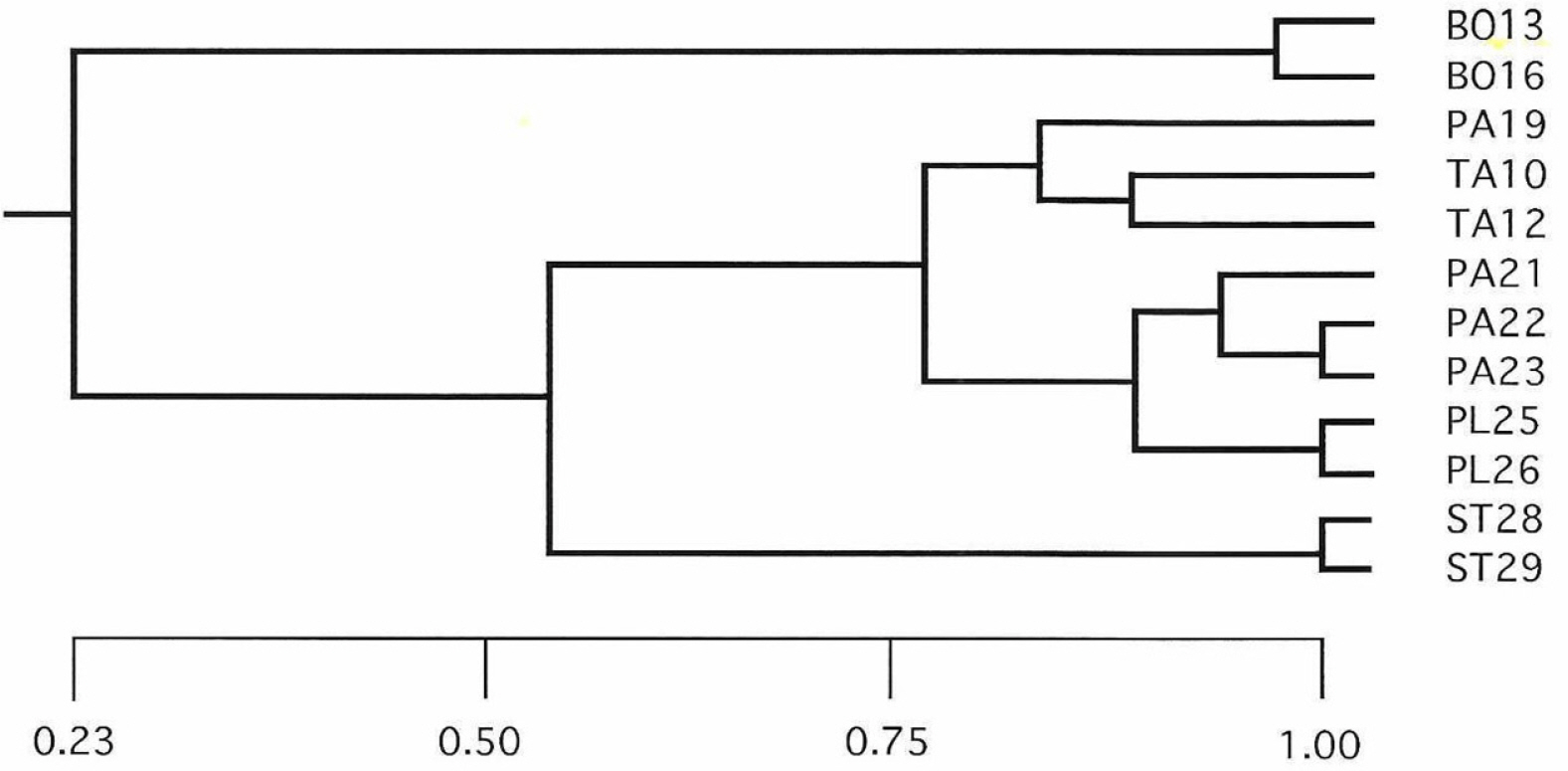
Unweighted pair-group method using arithmetic averages phenogram based on Nei’s (1978) genetic identity coefficients using 11 genetic loci from 12 populations representing five species of Australian Euphorbia sect. Eremophyton. The cophenetic correlation is 0.959. Species abbreviations: BO, E. boöphthona; PA, E. parvicaruncula; PL, E. planiticola; TA, E. tannensis; ST, E. stevenii.
Euphorbia stevenii (2n = 42, 84) has a pattern of fixed heterozygosity at the polymorphic loci: IDH-1, MDH-1, MDH-3, ME, 6PGD-2, PGI-1, PGI-2 and SKDH. Populations of E. boöphthona (2n = 56) examined expressed fixed heterozygosity for ME, 6PGD-2, PGI-1, PGI-2 and SKDH (Fig. 4). Fixed heterozygosity for most isozymes in E. stevenii and E. boöphthona strongly suggests that these two species are allopolyploids rather than autopolyploids.
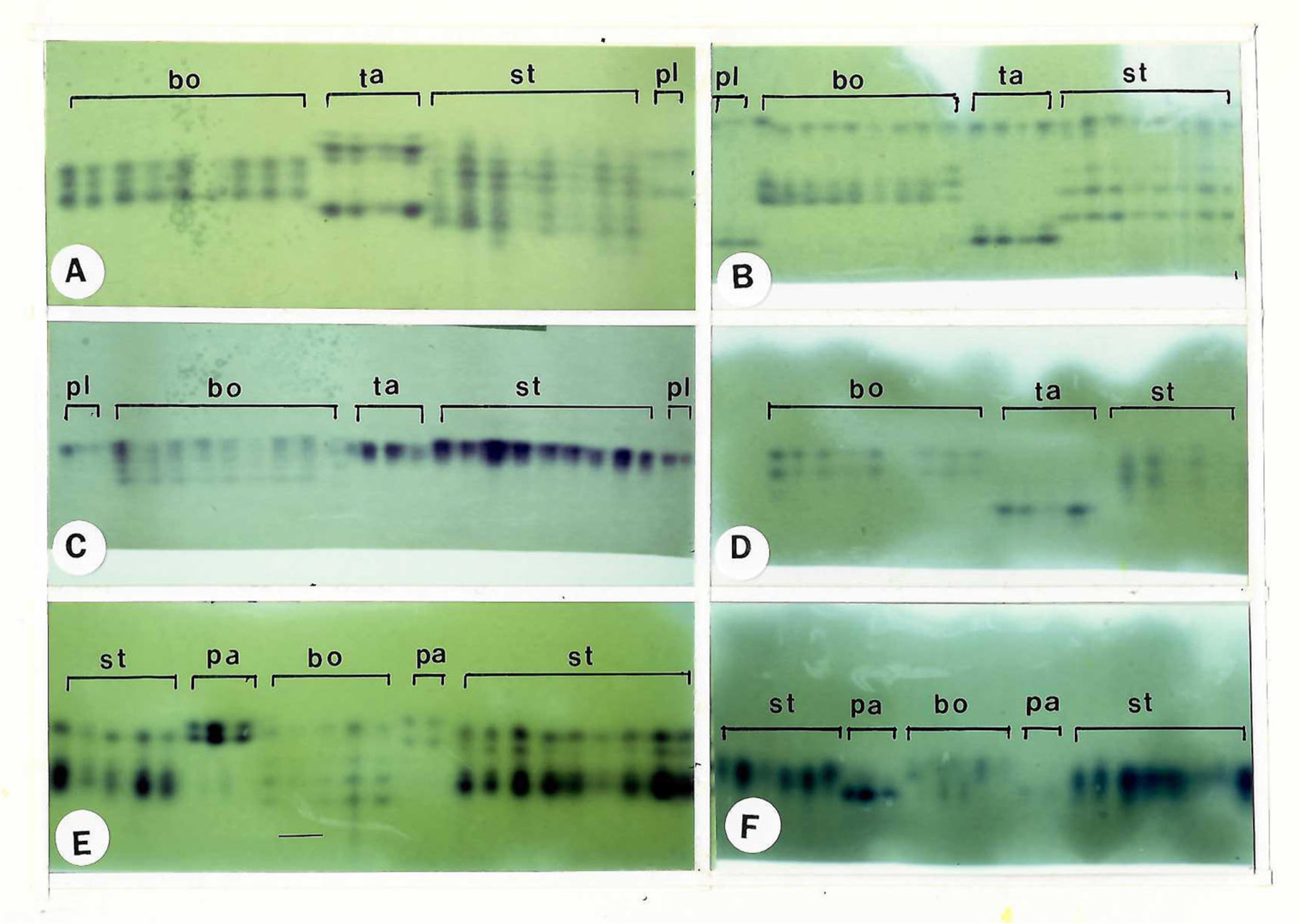
Photographs of Euphorbia isozyme gels. Enzyme abbreviations are indicated as below: A (malate dehydrogenase, MDH), B (phosphoglucose isomerase, PGI), C (malic enzyme, ME), D (shikimic dehydrogenase, SKDH), E (6-phosphogluconate dehydrogenase, 6PGD) and F (isocitrate dehydrogenase, IDH). Species abbreviations: BO, E. boöphthona; PA, E. parvicaruncula; PL, E. planiticola; TA, E. tannensis; ST, E. stevenii.
Euphorbia boöphthona has unique alleles such as ADH-1a, ADH-2a, MDH-3b, MEd and 6PGD-2d which are not present in any species of sect. Eremophyton. However, E. boöphthona and E. stevenii share the same alleles at IDH-1a, IDH-1b, 6PGD-1b, 6PGD-2b, PGI-2a and SKDHa (Table 3).
Euphorbia stevenii and diploid E. tannensis share the same alleles at MDH-1c, MDH-3c and MDH-3d. 6PGD-2c was only one unique allele present in E. stevenii (Table 3).
DISSCUSION
Australian species of Euphorbia sect. Eremophyton are herbaceous semi-succulent annual or biannual plants with a unique three (or two)-branching mainstem. Australian species of the section have basic number x = 7, and a polyploid complex with three diploids (2n = 14) and two polyploids, E. stevenii (2n = 42, 84) and E. boöphthona (2n = 56).
Hassall (1977) suggested that E. tannensis, the most widely distributed diploid, should be the most primitive species, and the rest of the species evolved into the interior of Australia through adaptive radiation. Although the polyploid origin of the two species have hypothesized cytologically, the origin and parentage of the species were not well documented genetically.
According to the analysis of genetic and morphological data, three diploid species which forming a cluster are considered to have differentiated recently, but two independent polyploid species are estimated to have differentiated relatively long ago. This hypothesis is also in well support with the seed characteristics of the two polyploids which differed in very distinctive external morphology compared to diploid species (Hassall, 1977; Weber, 1986).
Recent cytological studies showed that the polyploidy has played an important role in the species diversity of genus Euphorbia. Comparing the common occurrence of polyploid in the flowering plants (ca. 43–60%) (Grant, 1981), Hans (1973) reported that about 40 % of the Euphorbia species were polyploidy. Australian sect. Eremophyton is no exception, and two out of five are polyploid species, and it is presumed that polyploidization played an important role in adaptive radiation and colonization of diverse soil types of Australia (Hassall, 1977).
Origin of E. stevenii
Compared to widespread E. tannensis, E. stevenii has a relatively limited distribution over New South Wales, Northern Territory and Queensland of inland Australia, and is mainly found in clay beds of flood-plains after the rainy season. Morphologically E. stevenii is easily recognized by the ovoid and ecarunculate seed. Seed testa of the species shows dense papillose with tubercle-like papillae (Weber, 1986). It resembles the testa of E. boöphthona, but differs from the seed characters of three diploid species.
Hassall (1977) counted chromosome numbers of E. stevenii population which was collected from 80 km NE. of Windorah on Blackall Road, and showed that hexaploids (2n = 6x = 42) and dodecaploids (2n = 12x = 84) were found in the same population. In this study individuals from the same population (pop. 28, Table 1) were collected, and isozyme studies were conducted.
The presence of the fixed heterozygotes at eight of 11 loci strongly suggests that E. stevenii is allopolyploid species which was originated by the hybridization and chromosomal doubling between 4x and 2x species. Among the diploid species, E. tannensis shared the same alleles of E. stevenii at MDH-1c, MDH-3c and MDH-3d. Of the three diploids, E. tannensis is the most likely ancestor of E. stevenii, whereas it is difficult to consider E. parvicaruncula and E. planiticola as a possible ancestor of E. stevenii due to the absence of the fixed allele, MDH-3a, in E. stevenii. Since there are no extant tetraploid species in sect. Eremophyton, it is highly likely that this species was extinct. Thus, hexaploid E. stevenii probably has arisen through polyploidization between diploid E. tannensis and an extinct tetraploid (Fig. 5). In contrast, dodecaploid individuals (Hassall, 1977) probably might be originated by autopolyploidization which made by doubling the chromosome of hexaploid individuals of same population.

Origin of polyploid species of Australian Euphorbia sect. Eremophyton based on cytological (Hassall, 1976) and genetic studies. Some of the genomes are unknown or extinct at the diploid or tetraploid level. ‘X’ indicates hybridization and chromosomal doubling between species. Small letters designate genomes.
Since it is not certain whether dodecaploid individuals were included in this isozyme analysis, chromosome number should be first checked and isozyme studies should also be conducted to reveal the possibility of existence and origin of dodecaploid individuals. Because autopolyploids are morphologically indistinguishable from their progenitor, in genetic studies, precise sampling is necessary to avoid missing dodecaploid individuals (Coyne and Orr, 2004).
Origin of E. boöphthona
This species is widespread in the inner parts of Australia such as the Northern Territory and Western Australia, and occurs across wide range of soil types (Hassall, 1977). It is well recognized by the pendulous fruits and tuberculate seeds. Hassall (1977) counted chromosome numbers of E. boöphthona population which was collected from 78 km W. of Charleville, Qld., and this species occurred at the octoploid (2n = 8x = 56). In this study individuals from near the same population (pop. 13, Table 1) were collected and isozyme studies were conducted.
Populations of E. boöphthona examined expressed fixed heterozygosity for ME, 6PGD-2, PGI-1, PGI-2 and SKDH (Fig. 4). Fixed heterozygosity for five of 11 loci in E. boöphthona suggests that this species is allopolyploids.
Euphorbia boöphthona and E. stevenii shared the same alleles at IDH-1a, IDH-1b, 6PGD-1b, 6PGD-2b, PGI-2a and SKDHa, and it suggests that E. stevenii (2n = 42) might be a hexaploid ancestor of E. boöphthona, but, conversely, E. boöphthona cannot be an ancestor of E. stevenii because of the many orphan alleles in E. boöphthona. The surface pattern of E. boöphthona seeds is tuberculate, and is papillose with minute papillae between tubercles, and it resembles the seeds of E. stevenii which is densely papillose with minute papillae (Weber, 1986).
Euphorbia boöphthona has unique fixed alleles such as ADH-1a, ADH-2a, MDH-3b, MEd and 6PGD-2d which are not present in any species of sect. Eremophyton. The presence of many unique fixed alleles in E. boöphthona suggests the involvement of unknown or extinct diploid as another progenitor.
The most likely hypothesis for the origin of E. boöphthona is that it was made by hybridization and chromosomal doubling between an extinct diploid species and the hexaploid E. stevenii (Fig. 5).
Acknowledgements
This work was supported by grant from Korean Science and Engineering Foundation (KOSEF 981-0513-069-1).
Notes
CONFLICTS OF INTEREST
The author declares that there are no conflicts of interest.