국내 침입외래식물 사막갓(Brassica tournefortii; Brassicaceae)의 보고 및 잠재 분포 예측
New record and prediction of the potential distribution of the invasive alien species Brassica tournefortii (Brassicaceae) in Korea
Article information
Trans Abstract
The invasive alien species Brassica tournefortii Gouan (Brassicaceae) is herein reported for the first time in Korea, from Gunsan-si, Gochang-gun, and Jeju-si. Brassica tournefortii can easily be distinguished from B. juncea and B. napus by its dense stiff hairs at the base of the stem and leaves, basally and distally branched stems, partially dehiscent fruits, and seeds that become mucilaginous in the presence of moisture. Although some taxonomists have classified this species as belonging to Coincya Rouy based on its fruit and seed characteristics, the existence of one vein on the fruit valves and our maximum likelihood analysis using internal transcribed spacer sequences placed it in Brassica. Distribution data, photographs, and a description of B. tournefortii are presented herein. Moreover, potential changes in the distribution of B. tournefortii were predicted under different climate scenarios, but our analysis showed that the probability of the spreading of this species is low. Nevertheless, continuous monitoring is necessary for an accurate assessment. The results of the present study can be used to conduct an invasion risk assessment and can assist with the effective management of this invasive alien species.
Abstract
적 요:
침입외래식물인 Brassica tournefortii Gouan이 전북 군산시, 고창군, 제주도 제주시에서 발견되었다. 본 종 은 식물체 기부에 강모가 밀생하고, 줄기가 기부와 말단에서 분지하며, 열매는 성숙시 일부분만 열개되고, 종자는 수분이 있을 때 점성이 띠는 특징으로 배추속의 갓, 유채와 쉽게 구분된다. 일부 학자들은 B. tournefortii의 열매와 종자의 특징으로 Coincya Rouy 속에 속하는 분류군으로 취급하기도 하나, 열매의 과피 편 표면에 한개의 맥이 있는 특징과, ITS 구간 염기서열의 maximum-likelihood 분석 결과에 따라 배추속 식물로 분류하였다. 본 연구에서 B. tournefortii의 분포지, 화상자료 및 기재문을 제시하였으며, 또한 본 종의 잠재분포변 화가 기후 시나리오에 의해 예측되었다. 예측 결과, B. tournefortii의 국내 확산 가능성은 낮은 것으로 나타났지만, 정확한 판단을 하기 위해서는 지속적인 모니터링이 필요할 것으로 판단된다. 본 연구의 결과들은 B. tournefortii의 위험성 평가 및 효과적인 관리방안을 제시하기 위한 자료로 활용될 수 있을 것이다.
INTRODUCTION
Brassicaceae Burnett comprises 351 genera and 4,000 species worldwide, mostly distributed across the temperate regions of the northern and southern hemispheres (Abdelhameed et al., 2020). The family generally has typical morphological characteristics: tetradynamous stamens, four cruciform or butterfly-like petals, and capsules with two locules divided by septa (Beilstein et al., 2006).
Brassicaceae members having conduplicate cotyledons and the presence or absence of simple hairs are classified into tribe Brassiceae, and some of those with dehiscent fruits at maturity are further subdivided into subtribe Brassicinae (Kadereit, 1994). Meanwhile, most members of Brassica have completely dehiscent fruits at maturity, whereas some plants with partially dehiscent fruits can even be classified as Coincya Rouy (Leadlay and Heywood, 1990; Kadereit, 1994; Al-Shehbaz et al., 2006a, 2006b; Nagpal et al., 2008; Al-Shehbaz, 2012). Owing to the highly variable morphological characters, however, taxonomy of some taxa within Brassica is debatable. Therefore, comprehensive studies for both morphology and molecular patterns are required to elucidate the taxonomic delimitations of some taxa.
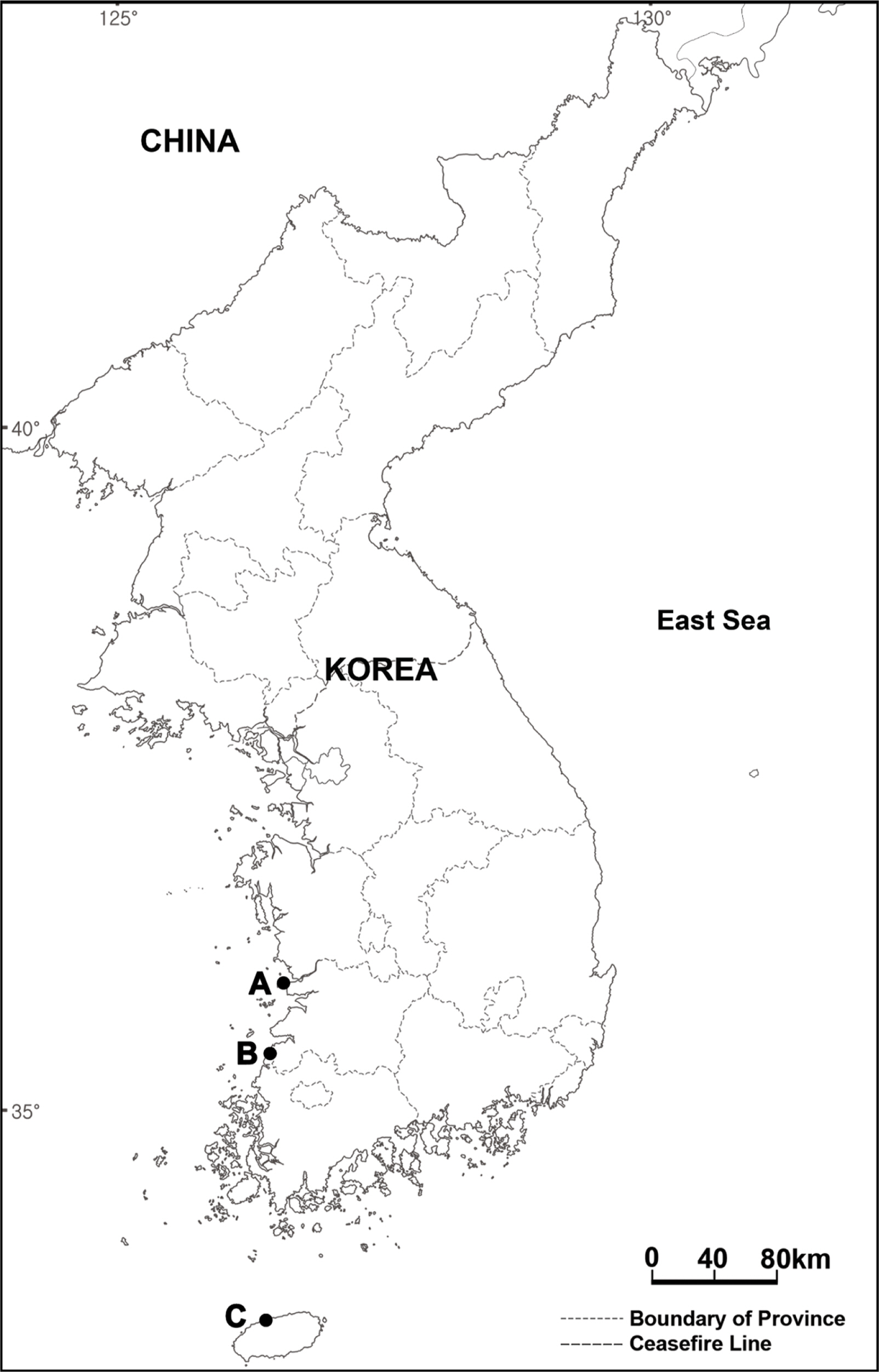
In the present study, we report the invasive alien species Brassica tournefortii Gouan for the first time in South Korea. It was first detected in Gunsan-si of Jeollabuk-do in 2018, after which it was also found in Gochang-gun of Jeollabuk-do and Jeju-si of Jeju-do in 2020 (Fig. 1). Moreover, this plant has been treated as either B. tournefortii or Coincya tournefortii (Gouan) Alcaraz, T. E. Díaz, Rivas Mart. & Sánchez-Gómez (Alcaraz Ariza et al., 1989; The Plant List, 2013; POWO, 2022). Hence, its taxonomic position was reviewed by comparing its morphological characteristics with those of related species in South Korea and by using nuclear ribosomal DNA internal transcribed spacer (ITS) sequence data—which is a useful marker for phylogenetic analysis of the Brassicaceae (Álvarez and Wendel, 2003; Qi et al., 2007; Warwick et al., 2010; Salariato et al., 2013)—obtained from collected materials in Gochang-gun and registered in the National Center for Biotechnology Information (NCBI) database.
The species’ potential distribution under climate change scenarios was also modeled based on the current species distribution information (GPS data) in Gunsan-si, Gochang-gun, and Jeju-si. Invasive alien plant tend to have high dispersal abilities, rapid growth with short generation times, and high tolerance of broad environmental conditions. Therefore, climate-related changes will almost certainly lead to changes in the distribution of alien plant species, as their populations respond to variability and changes in temperature, precipitation, and biotic interactions. Predicting how invasive species will respond under potential climate change scenarios is difficult but understanding alien plant species’ current and future distribution patterns under different climate scenarios may provide primary data that can be used for invasion risk assessment (Moran and Alexander, 2014).
The findings of the present study elucidate the identification of Brassica in South Korea, besides providing data on the distribution of B. tournefortii, which can be a fundamental resource for the invasion risk assessment and effective management of this alien plant species.
MATERIALS AND METHODS
Morphological analysis
Living, dry, and immersed specimens collected in 2018–2021 from Gunsan-si and Gochang-gun of Jeollabuk-do and Jeju-si of Jeju-do island were studied. Morphological features were analyzed under a digital camera (Nikon D810 + Nikon 105 mm AF Micro-Nikkor, Tokyo, Japan) and measured using a digital Vernier caliper (Mitutoyo 500-196-30 absolute digimatic Vernier caliper, Tokyo, Japan). The obtained results were compared with descriptions and illustrations of related genera and species from previous studies (Leadlay and Heywood, 1990; Kadereit, 1994; Al-Shehbaz et al., 2006a, 2006b; Nagpal et al., 2008; Al-Shehbaz, 2012). The examined material has been deposited in the Korea National Arboretum (KH).
Molecular genetic analysis
DNA extraction and PCR
Three samples of fresh leaves of B. tournefortii collected from Gochang-gun in 2021 were stored in silica gel and completely dried. A tissuelyser (Mixer Mill MM 200, Retsch, Haan, Germany) was used for pulverization, and total genomic DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). The PCR mix consisted of 1 μL of DNA (extracted using the AccuPower PCR PreMix; Bioneer, Daejeon, Korea) with 10 μmol of ITS1 (forward) and ITS4 (reverse) primers with 1 μL and 17 μL of distilled water, respectively. PCR was performed in a DNA thermal cycler (Biometra, Whatman Co., Göttingen, Germany), with 35 cycles of 5 min at 94°C, 1 min at 94°C, 1 min at 54°C, and 1 min at 72°C, followed by 7 min at 72°C for fixation. PCR product purification and sequencing were performed by Macrogen, Co., Ltd. (Seoul, Korea).
Phylogenetic analysis
The ITS sequence data of B. tournefortii from Gochang-gun were compared with ITS sequences of related genera and species obtained from the NCBI database. In total, 20 species of Brassica and four of Coincya were included. Sisymbrium officinale (L.) Scop. and Barbarea orthoceras Ledeb. were used as outgroups. The sequences were aligned using MAFFT in Geneious ver. 8.0.5 (https://www.geneious.com). Maximum likelihood (ML) analysis was performed using 5,000 bootstraps and the STR + R2 model in the IQ-TREE 1.6.8 software (http://www.iqtree.org).
Potential distribution analysis
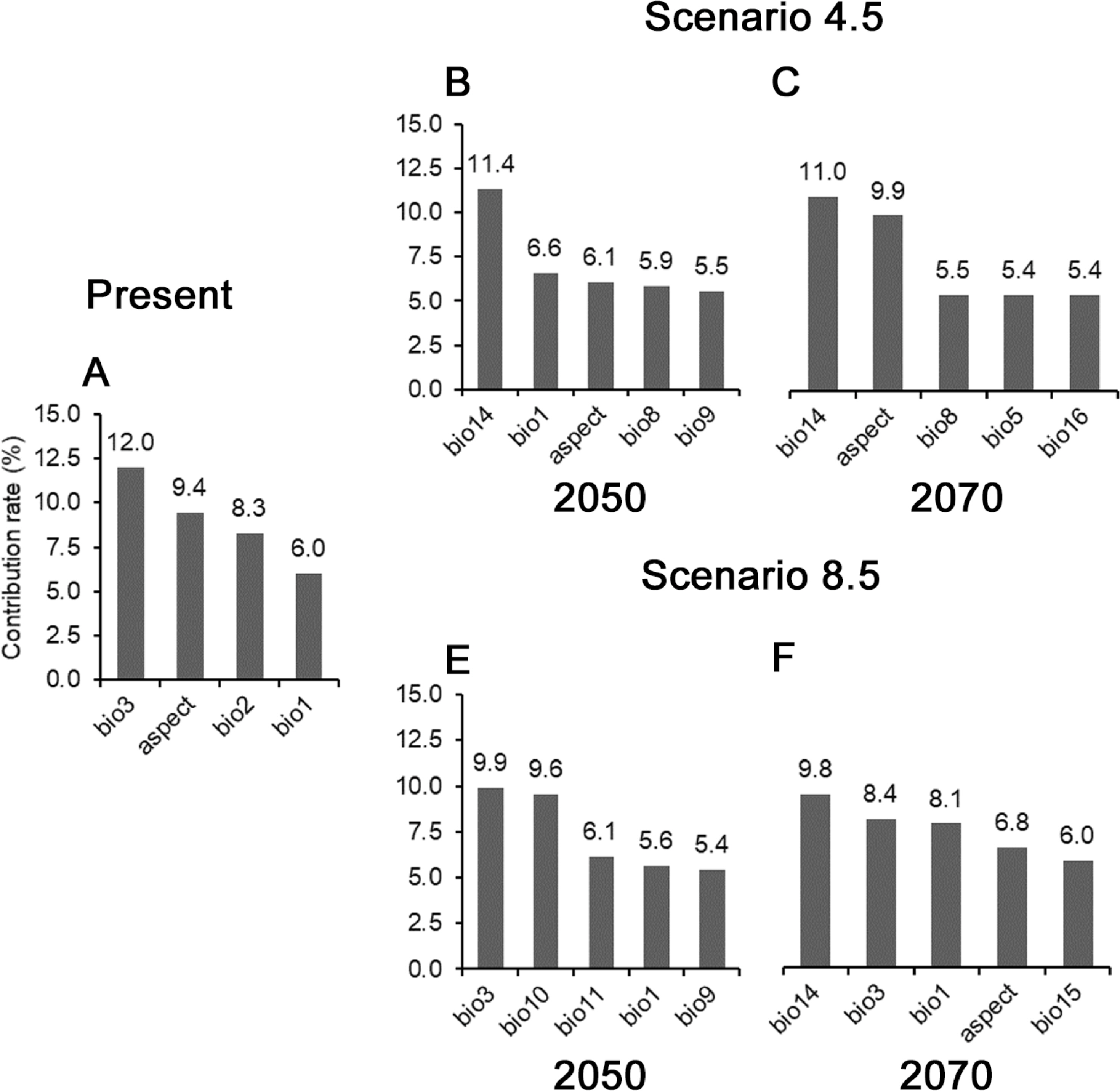
To predict the projection and change of potential distribution according to the current and climate change scenarios of B. tournefortii, the distribution coordinates of each B. tournefortii individual were collected from Gochang-gun, Gunsan-si, and Jeju-si. For predictive analysis, the SSDM package was used in the RStudio program based on the collected distribution coordinates (Schmitt et al., 2017). The Ensemble Species Distribution Models (ESDMs) fits nine algorithms, explores the prediction range across different species distribution models (SDMs), and then finds consensus among the SDM predictions (Table 1). The 23 environmental factors most commonly applied were used (e.g., mean annual temperature and precipitation) (Table 2).

The 23 abiotic variables used in the species distribution modeling of Brassica tournefortii Gouan and their respective descriptions.
To determine the potential distribution of B. tournefortii in South Korea under future climate trajectories, we applied the 4.5 and 8.5 scenarios of the Representative Concentration Pathway (RCP) for three different periods (current, 2050, and 2070). The RCP 4.5 scenario, as presented in the 5th IPCC report, is a stabilization scenario in which the total radiative forcing reaches 4.5W/m2 by 2100 and stabilizes due to the employment of various technologies and strategies to reduce greenhouse gas emissions (Shrestha and Bawa, 2014). The RCP 8.5 scenario predicts that terrestrial mean temperature and precipitation will increase by 4.8°C and 6.0%, respectively, if there is a continued lack of efforts to reduce greenhouse gas emissions (IPCC, 2013).
By presenting only the potential distribution and the significantly correlated factors in our analysis according to the current and climate change scenarios, rather than comparing models, the focus was on predicting changes in the distribution of an alien species.
RESULTS
Morphological analysis
The basal leaves of young Brassica tournefortii have deep dissected lobes and are densely hirsute (Fig. 2B, F, G), closely resembling the radical leaves of young Cirsium japonicum Fisch. ex DC. var. maackii (Maxim.) Matsum. In addition, the flowering plant is similar to those of the species of genera Sisymbrium Burnett on Raphanus L. (Fig. 2B, I, J), but the characteristic of the fruit, i.e., dehiscence into two valves along the suture at maturity, confirms that the species belongs to Brassica (Fig. 2O) (Kadereit, 1994; Al-Shehbaz et al., 2006a, 2006b; Oh, 2007).

Brassica tournefortii Gouan in Korea. A. Habitat in Gochang-gun. B. Plant. C. Root. D. Basal stem E. Upper stem. F. Adaxial basal leaves. G. Abaxial basal leaves. H. Cauline leaves (left, adaxial; right, abaxial). I. Inflorescence. J. Flower. K. Sepals. L. Petals. M. Stamens. N. Nectar glands. O. Fruits. P. Seeds. Q. Seed surface.
Apart from these features, B. tournefortii can also be easily distinguished from the two other Brassica species, B. juncea (L.) Czern. and B. napus L., by many branches basally and distally on the stem, hairs on proximal stems, remain seeds in indehiscenced fruit beak, seeds with mucilaginous seed coats in the presence of moisture (Fig. 2B, D, P, Q) (Kadereit, 1994; Al-Shehbaz et al., 2006a, 2006b; Oh, 2007).
Molecular genetic analysis
In total, 28 ITS sequences were obtained from two accessions of B. tournefortii and the 24 related taxa (with two outgroups: Barbarea orthoceras and Sisymbrium officinale) (Fig. 3). The total alignment length of the ITS was 580 base pairs, and 74 variable sites were found. The identical sites were 340 base pairs (58.6%). The overall GC ratio was 53.5% for ITS.
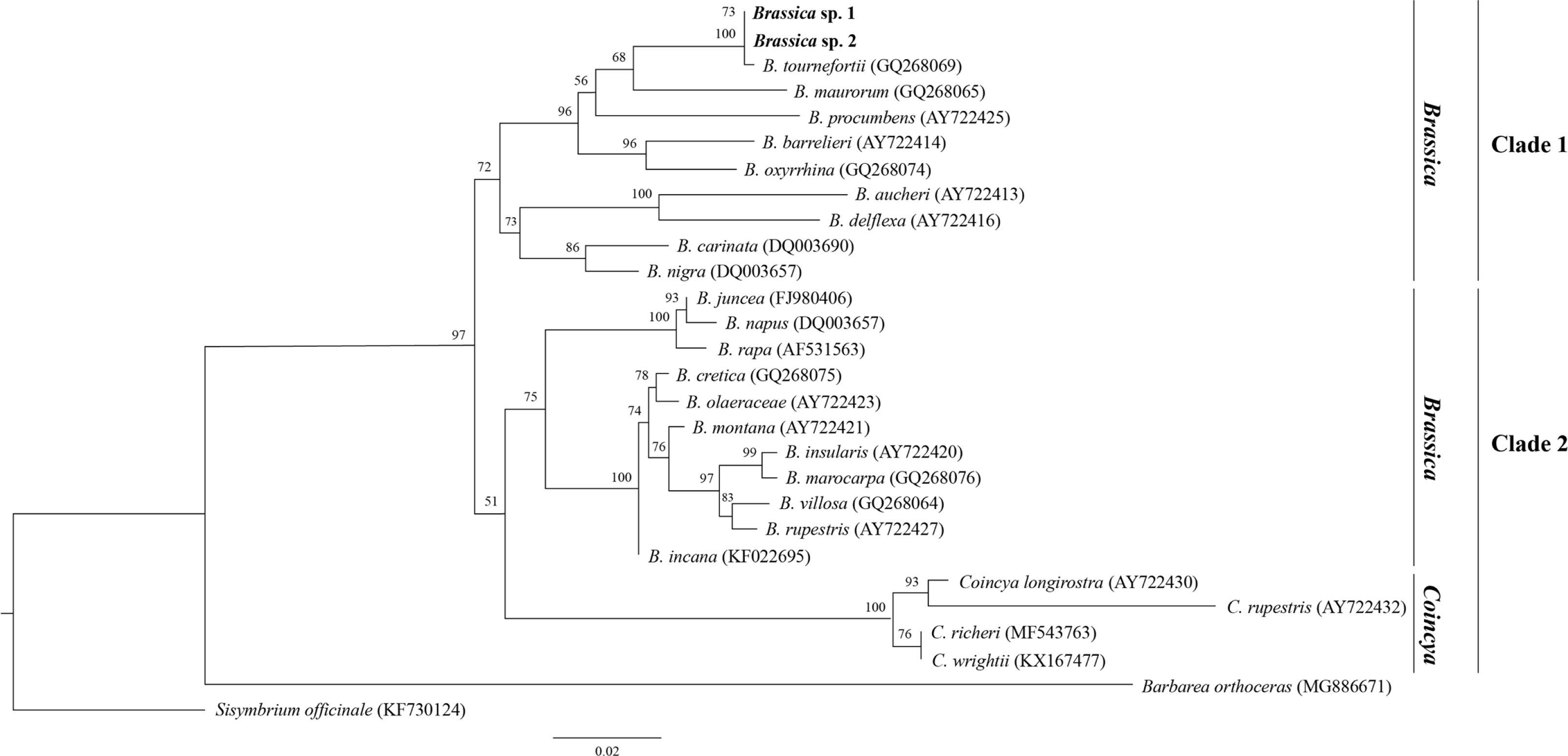
Maximum likelihood (ML) tree of internal transcribed spacer sequence data of Brassica tournefortii Gouan and related species. Numbers above branches indicate bootstrap support values.
In ML trees, all 26 accessions of Brassica and Coincya species formed a monophyletic group (bootstrap value; BS = 97), excluding the outgroups (Fig. 3). Members of Brassica were separated into two clades, one of which formed a cluster with Coincya, even though overall node support was not robust (BS = 51). The identity of the Gochang-gun individual was B. tournefortii, as strongly supported by BS (BS = 100).
Potential distribution analysis
The potential distribution of Brassica tournefortii in the current climate condition was predicted, including the coastal regions where the plants were recorded (Gunsan-si, Gochang-gun, and Jeju-si), covering 2.2% of the total area of South Korea (Fig. 4A). In the RCP 4.5 scenario, the distribution was projected to reduce to 2.2% by 2050 and to 1.6% by 2070 (Fig. 4B, C). In both cases, bio 14 (precipitation of the driest month) was assessed as a significant factor (Fig. 5B, C). In this scenario, compared to the result that predicted the current climate condition, the probability of distribution of B. tournefortii decreased in Gochang-gun and Jeju-si but increased on the islands of the Yellow Sea. However, in general, the area of potential distribution decreased with time. In the RCP 8.5 scenario, the potential distribution was projected to increase to 1.5% by 2050, with the contribution of bio 3 (isothermality), and to 1.9% by 2070, influenced by bio 14 (Figs. 4D, E, 5D, E). In this scenario, the probability of distribution of B. tournefortii decreased in Gunsan-si and Gochang-gun and then increased in Gunsan-si, Gochang-gun, and Jeju-si by 2070.
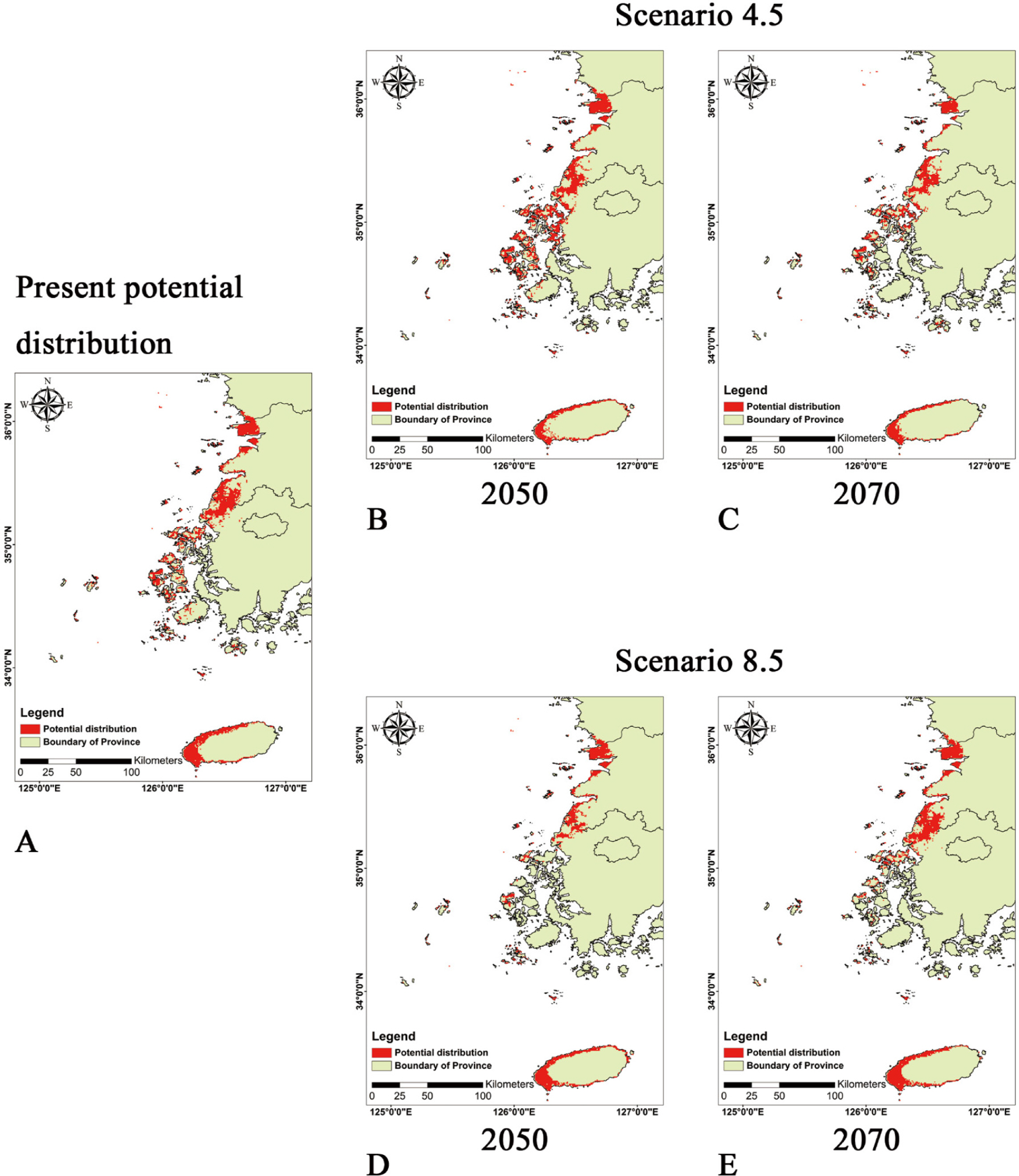
Potential distribution of Brassica tournefortii Gouan under the current scenario and climate change scenarios. A. Present. B. RCP4.5 2050. C. RCP4.5 2070. D. RCP8.5 2050. E. RCP8.5 2070.
DISCUSSION
Brassica tournefortii, unlike other Brassica species in South Korea, has pinnatisect basal leaves that are densely hirsute. Thus, at young stages, it is sometimes confused with either Sisymbrium or Raphanus species (Fig. 2B, F, G). However, they are very different in the presence of bracts on racemes, fruit shape, and cotyledon type of seeds (Kadereit, 1994; Al-Shehbaz et al., 2006a, 2006b; Oh, 2007). B. tournefortii and Raphanus species have racemes without bracts, globose seeds, and conduplicate cotyledons, whereas Sisymbrium species have racemes with bracts, flattened seeds, and incumbent cotyledons (Fig. 2B, P, Q) (Kadereit, 1994; Al-Shehbaz et al., 2006a, 2006b; Oh, 2007) (Fig. 2P, Q). Conversely, B. tournefortii can be distinguished from Raphanus species by morphological features such as yellow or light yellowish petals (vs. Raphanus, which has purple, pink, yellow, or white petals) and dehiscent fruits along the suture (vs. Raphanus, which has indehiscent fruits, often breaking transversely into segments fruits) (Fig. 2J, L, O) (Kadereit, 1994; Al-Shehbaz et al., 2006a, 2006b; Oh, 2007).
The two other Brassica species from South Korea, B. juncea and B. napus, have basal leaves that are not pinnatisect but lyrate to pinnatifid and are glabrous or sparsely hirsute; therefore, B. tournefortii can be easily distinguished from these two species. Moreover, both B. juncea and B. napus are branched at the upper part of the stem, have completely dehiscent fruits at maturity, with seed coats that do not turn mucilaginous when in contact with water, but B. tournefortii has stems branched basally and distally, partly indehiscent fruit beaks, and seed coats that become mucilaginous with moisture (Fig. 2B, D, P, Q) (Al-Shehbaz et al., 2006a, 2006b; Oh, 2007).
Meanwhile, one Brassicaceae member, the genus Coincya, shares a typical trait of partly indehiscent fruits; thus, some taxonomists consider this species as belonging to Coincya (Leadlay and Heywood, 1990; Kadereit, 1994; Al-Shehbaz et al., 2006a, 2006b; Nagpal et al., 2008; Al-Shehbaz, 2012). However, the existence of one vein on the fruit valves reveals that B. tournefortii really belongs to Brassica, as Coincya has three or more veins (Leadlay and Heywood, 1990; Al-Shehbaz et al., 2006a) (Fig. 2O). Furthermore, our ML tree showed that Coincya is not monophyletic because it clusters with the other group of Brassica, and B. tournefortii is more closely related to Brassica than to Coincya (Fig. 3). Therefore, we herein classify it as a Brassica member.
Brassica tournefortii is native to North Africa and countries near the Caribbean Sea, Central Asia, and Western India (Malusa et al., 2003; Dahlin et al., 2012; Abd El-Gawad, 2014; Curtis and Bradley, 2015; Winkler et al., 2019). The species was mainly known to have naturalized and spread to Australia and southern parts of the U.S.A. (Trader et al., 2006; Barrows et al., 2009; Dahlin et al., 2012; Abd El-Gawad, 2014; VanTassel et al., 2014). It was recently reported to have been introduced into China and Japan (Curtis and Bradley, 2015; Winkler et al., 2019; Kraus et al., 2020). In the U.S.A., B. tournefortii is presumed to have been introduced through date palm import from the Middle East in 1927 (Sánchez-Flores, 2007; Marushia et al., 2012; Winkler et al., 2019).
Brassica tournefortii usually grows on coastlines and along roads and railways (Trader et al., 2006; Lillian, 2017; Rahmani et al., 2020). As it shows strong resistance to drought stress, a desert ecosystem is suitable for its growth (Choudhary and Joshi, 2001; Abd El-Gawad, 2014; Winkler et al., 2018; Rahmani et al., 2020). The lifecycle of B. tounefortii is short, with two to three generations within a year; it produces thousands of seeds at a time, which is advantageous for dispersion; and the seeds have a high rate of gemination. These factors make this species highly invasive (Bangle et al., 2008; Dahlin et al., 2012; Abd El-Gawad, 2014; Curtis and Bradley, 2015). B. tournefortii seeds can remain dormant in an environment unfavorable for germination, and they can withstand flooding as the seed coats become mucilaginous when supplied with excessive moisture (Sánchez-Flores, 2007; Bangle et al., 2008; Dahlin et al., 2012; Abd El-Gawad, 2014; Berry et al., 2014; Curtis and Bradley, 2015). These mechanisms also allow the seeds to adhere to animals, further facilitating dispersion (Li et al., 2015; Winkler et al., 2018, 2019).
The spread of B. tournefortii is considered a chief factor in the reduction of biodiversity in desert ecosystems. In desert regions in the south of the U.S.A., the increase in annual precipitation and transport infrastructure has increased the spread of B. tournefortii as compared to the early distribution regions of the species (Malusa et al., 2003; Schiermeier, 2005; Barrows et al., 2009; Marushia, 2009; Dahlin et al., 2012; Marushia et al., 2012; VanTassel et al., 2014; Winkler et al., 2019), which in turn has caused it to monopolize resources previously used by native plants and disturb the habitats of small animals such as lizards (Trader et al., 2006; Sánchez-Flores, 2007; Bangle et al., 2008; Dahlin et al., 2012; Abd El-Gawad, 2014; Berry et al., 2014; Curtis and Bradley, 2015; Rahmani et al., 2020). The spread of this invasive alien plant was found to have also influenced the frequency of fires in the desert (Steers, 2008), prompting studies worldwide to identify the growth, ecological, and physiological characteristics of the species to control its dispersion and invasion (Chauhan et al., 2006; Trader et al., 2006; Marushia, 2009; Abd El-Gawad, 2014; Alfaro and Marshall, 2019). In particular, notable efforts have been made in the U.S.A., where B. tournefortii was designated as an invasive alien plant to prevent its spread and damage (Chauhan et al., 2006; Lillian, 2017; Kraus et al., 2020).
Brassica tournefortii, which inhabits in arid and semi-arid arears, showed narrow range of potential distribution according to the current environmental envelope in South Korea, and it was evaluated that the potential distribution area shown to not increase under the climate change scenarios (Fig. 4). However, the studies of Winkler et al. (2018, 2019) revealed that B. tournefortii exhibits population variability in germination and growth according to the local seasonal precipitation. Therefore, it is assumed that this species can adopt to trait of local environment, and increasing reproduction and competitiveness with native species. In Korea, ecological studies have not been conducted to date on B. tournefortii in the three aforementioned regions under human interference and specific environmental conditions (e.g., nutrient distribution; seed production; vegetation; competing species; soil; and the biological interaction network, including herbivores). Instead, considering the studies on the species’ dispersion in the U.S.A. and its growth and ecological characteristics, the probability of rapid spread in South Korea can be regarded as extremely low due to the lack of a broad prairie or desert area. The potential distribution of B. tournefortii was assessed that this species’ dispersion depends on spring and autumn precipitation, with little increase predicted for the concerned regions (Figs. 4, 5).
It is assumed that these results arise from B. tournefortii recent introduction to Korea (first detection in Gunsan-si in 2018) and main distribution along the coast. However, recently introduced alien plants ensure sustainability by forming a soil seed bank in a given region and spreading to surrounding regions, and these may be supported by macroscale climate factors and complex interactions in the ecosystem (Lim et al., 2020; Park and Choi, 2020). Under low conditions of inter- and intra-specific resource competition, B. tournefortii produces a high amount of seeds (Trader et al., 2006). The species’ characteristic onset in early spring, when no other species are growing, may enable the dispersion of B. tournefortii from the site of introduction in South Korea. This survival strategy of B. tournefortii may enable a continued invasion in future climate change scenarios through self-fertilization and propagation, and it is expected to compete with native species. In addition, future spread to island and coastal regions, cultivated lands, disturbance zones with sand-rich soil, and agricultural lands with environments similar to the current habitats is possible. Thus, preparation against the uncontrolled spread of the species, especially under anthropogenic pressure, is necessary. A possible measure for preventing the species’ migration toward inland regions is the removal of B. tournefortii plants along sand bed channels and dry lands at banks.
SDM analysis using distribution information of recently introduced alien plants revealed narrow predicted areas because a narrow environmental factor distribution range was used. Although the approach according to the current conditions and climate change scenarios has these limitations, it clearly has the advantage of rationally extracting uncertain distribution conditions at the beginning of the introduction of exotic plants. The results of this study will be helpful in various studies and vegetation management activities necessary for exploring the distribution of exotic plants and understanding species characteristics.
Taxonomic treatment
Brassica tournefortii Gouan, Ill. Observ. Bot. 44, t. 20A, 1773; Erucastrum tournefortii (Gouan) Link, Handbuch 2: 317, 1831; Coincya tournefortii (Gouan) Alcaraz, T.E. Díaz, Rivas Mart. & Sánchez-Gómez, Itinera Geobot. 2: 108, 1989. (Fig. 2)
Herbs annual, erect, .0.1–1 m tall. Stems branched basally and distally, green or purple, hirsute proximally. Basal leaves rosettes, persistent; petiole 1.0–4.0 cm; blades lyrate, deeply pinnatisect, 11.0–20.0 × 4.0–6.0 cm, with lobes 4–8 each side, margin serrate to dentate, hirsute; terminal leaflets 2.0–3.2 × 2.0–2.5 cm; lateral leaflets 2.0–3.0 × 1.0–2.0 cm. Cauline leaves alternate, subsessile or petiolate; petiole less than 2.0 cm; blades oblanceolate, 3.0–5.0 × 2.0–3.0 cm, margins serrate, base tapered, not auriculate or amplexicaul, reduced in size distally and densely hirsute. Inflorescence raceme, elongate rachis with flowering. Flowers bisexual, actinomorphic; pedicel 3.6–4.5 cm long; sepals 4, erect or spread, elliptic, central sepals 3.6–3.8 × 1.4–1.5 mm, lateral sepals 3.1–3.7 × 1.1–1.3 mm, apex obtuse, base saccate, exterior usually purplish, 1–2 bristles on upper; petals 4, oblanceolate or spatulate, 5.5–7.0 × 1.5–2.0 mm wide, apex round or obtuse, light yellow with dark yellow or brown veins on surface; stamens 6, tetradynamous, linear, white; central filaments 2.5–3.3 mm long, lateral filaments 1.0–1.6 mm long; anthers oblong, yellow, ones of central filament 1.4–1.6 × 0.6–0.8 mm, ones of lateral filaments 1.2–1.4 × 0.5–0.7 mm; nectary glands 4, median glands 2, lateral glands 2; pistil 2.9–3.3 × 0.6–0.8 mm, 2-loculed; ovules 5–8 per locule; styles 1.3–1.7 mm long; stigma capitate, 0.5–0.7 mm wide. Fruit capsule, dehiscent, spreading or ascending, torulose, cylindric, 4.5–6.0 cm long, 2.1–2.5 wide; valves 2 with a prominent midvein; terminal segment 1–3-seeded, indehiscent; stalk 7.7–15.8 mm long. Seeds uniseriate, globose, light reddish brown to black, 2.1–2.5 mm diam; seed coat reticulate, mucilaginous when wetted; cotyledons conduplicate.
Korean name: Sa-mak-gat (사막갓)
English name: Sahara mustard, African mustard.
Flowering: February–April, Fruiting: April–May.
Distribution: North Africa, countries near the Caribbean Sea, Central Asia, Western India, Australia, and south-western regions of the U.S.A., China, Japan, and Korea.
Distribution in South Korea: Gunsan-si, Gochang-gun, Jeju-si.
Examined specimen: GREECE. Chalkidiki: Flojita, 11 Apr 2003, B 10 0123089 (B); N Nea Moudania, 15 May 2019, B 10 1081147, B 10 1081148 (B). KOREA. Jeollabuk-do: Gunsan-si, Osikdo-dong, 13 May 2018, NMJ201805131, NMJ201805132, NMJ201805133 (KH); Gochang-gun, Jaryong-ri, 21 Mar 2021, KJS0708 (KH). Jeju-do: Jeju-si, Iho 1-dong, 13 Mar 2021, LSG0006 (KH); 21 Apr 2021, KES2104211, KES2104211 (KH). U.S.A. California: Riverside, 9 Mar 1995, 00043448 (NY). Nevada: Clark, 3 Apr 1998, 03173361 (NY). Utah: Washington, 11 Jan 1999, 00404781 (NY). Arizona: Mohave, 8 Apr 2000, 00584551 (NY).
A key to genera and species related to Brassica L. in Korea
1. Racemes bracteate; seed flattened; cotyledons incumbent · ···································· Sisymbrium Burnett (노랑장대속)
1. Racemes ebracteate; seed globose; cotyledons conduplicate
2. Petals purple, pink, yellow or white; fruits indehiscent, often breaking transversely into segments ·················· ······················································· Raphanus L. (무속)
2. Petals yellow or light yellow; fruits dehiscent along the suture
3. Vein of fruit valves more than 3 ··· Coincya Rouy
3. Vein of fruit valves 1 ········· Brassica L. (배추속)
4. Cauline leaves sessile, base auriculate or amplexicaul ························· B. napus L. (유채)
4. Cauline leaves sessile or petiolate, base not auriculate or amplexicaul
5. Plant glabrous; stems branched distally; basal leaves not persistent, blade pinnatifid to pinnately lobed, lobes 1–3 each side; fruit completely dehiscent at maturity; seed coat not mucilaginous when wetted ································· ·················································· B. juncea (L.) Czern. (갓)
5. Plant hirsute proximally; stems branched basally and distally; basal leaves persistent, blade deeply pinnatisect, lobes 4–8 each side; fruit partly indehiscent at mature; seeds coat mucilaginous when wetted ······························· ······················································ B. tournefortii (사막갓)
Acknowledgements
This study was supported by the project “Research on landscape genetics and distribution of invasive alien plants in Korea according to climate change (KNA1-2-39, 21-2)” funded by the Korea National Arboretum. We sincerely thank Dr. Yong-Chan Cho and Dr. Yoon-Young Kim for their constructive criticism and valuable comments, which were of great help in revising the manuscript.
Notes
Conflict of Interest
The authors declare that there are no conflicts of interest.