Morphometric analysis of the Daphne kiusiana complex (Thymelaeaceae) using digitized herbarium specimens
Article information
Abstract
Daphne kiusiana is an evergreen shrub with dense head-like umbels of white flowers distributed in southern Korea, Japan, China, and Taiwan. Plants in China and Taiwan are recognized as var. atrocaulis by having a dark purple stem, elliptic leaves, and persistent bracts. Recently, plants on Jejudo Island were segregated as a separate species, D. jejudoensis, given their elliptic leaves with an acuminate apex, a long hypanthium and sepals, and a glabrous hypanthium. Morphological variations of three closely related taxa, the D. kiusiana complex, were investigated across the distributional range to clarify the taxonomic delimitation of members of the complex. Twelve characters of the leaf and flower were measured from digitized herbarium specimens using the image analysis program ImageJ and were included in a morphometric analysis, the results of which indicate that the level of variation in the characters is very high. The results of a principal component analysis weakly separated D. jejudoensis from D. kiusiana according to their floral characteristics, such as a longer, glabrous hypanthium, and larger sepals. However, some individuals of D. kiusiana, particularly those from Bigeumdo Island, were included in D. jejudoensis. Recognition of D. kiusiana var. atrocaulis based on the leaf shape was not supported in the analysis, and D. jejudoensis may be recognized as a variety of D. kiusiana. Our morphometric analysis shows that digitized images of herbarium specimens could be useful and an additional method by which to investigate more diverse specimens.
INTRODUCTION
The genus Daphne L., consisting of about 95 species in Europe, northern Africa, and eastern Asia, belongs to the tribe Daphneae Meissn. in Thymelaeaceae and includes many economically important species (Herber, 2003; White, 2006; Oh and Hong, 2015). Species of Daphne are widely cultivated as ornamental plants for their colorful flowers and delicious scent. One such example is D. odora Thunb., perhaps the most widely cultivated Daphne species in eastern Asia and Europe, with many cultivars. Daphne kiusiana Miq. is morphologically closely related to D. odora and can be characterized by having glabrous stems, persistent and glabrous leaves, a terminal head-like umbel with caducous bracts on the previous year’s branch, and white, tetramerous flowers with cup-shaped nectary disks (Oh and Hong, 2015). Daphne odora is easily distinguished from D. kiusiana by having reddish pink flowers (strongly so on the outer surface of the hypanthium). While the native distribution and the origin of D. odora are unknown, D. kiusiana is distributed in China, Taiwan, Korea, and Japan (Ohwi, 1965; Lee, 2003; Wang et al., 2007).
Two varieties have been recognized in D. kiusiana: plants in Korea and Japan are recognized as D. kiusiana var. kiusiana (Ohwi, 1965; Oh and Hong, 2015) and those in China and Taiwan as D. kiusiana var. atrocaulis (Rehder) F. Maek. (Wang et al., 2007). In the taxonomic treatment of Thymelaeaceae in China, Rehder (1916) stated that var. atrocaulis is similar to var. kiusiana, but the former differs by having caducous bracts, thicker leaves, and dark purple stems. In Korea, D. kiusiana var. kiusiana is distributed on mountain slopes on offshore islands in the southwest (Bigeumdo and Uido Islands) and in the southeast (Geojedo Island) (Fig. 1A, B). Plants previously identified as D. kiusiana var. kiusiana in the Gotjawal forest on Jejudo Island have recently been segregated as a distinct species, D. jejudoensis M. Kim (Lee et al., 2013) (Fig. 1C). This new species was distinguished from D. kiusiana by having elliptic leaves, a glabrous hypanthium, and a long hypanthium and sepals (Lee et al., 2013).

Photographs of D. kiusiana var. kiusiana and D. jejudoensis. A. D. kiusiana var. kiusiana on Geojedo; B. D. kiusiana var. kiusiana on Bigeumdo; C. D. jejudoensis on Jejudo. (Photo credit: A, C: Sang-Hun Oh, B: Dong-Hyuk Lee)
However, these morphological characteristics delimiting each taxon show a wide range of variation within and among populations (Lee and Oh, 2017). Considering that an assessment of the morphological variations among species and varieties as well as across distributional ranges of temperate evergreen forests has not been conducted, a comprehensive analysis of the morphological variations could help to circumscribe the species. The D. kiusiana complex here is defined to include the two varieties of D. kiusiana and D. jejudoensis.
A virtual herbarium is a herbarium that houses digitized collections and provides various forms of information attached to the collections to broader users (Barkworth and Murrell, 2012; Monfils et al., 2017). The idea of the virtual herbarium was initially advanced in the U.S.A. in the 1990s, and since then individual herbaria and a consortium of multiple herbaria have led to the creation of virtual herbaria that serve as additional portals for botanical research and education. Virtual herbaria are now commonly available in most major herbaria in many regions, i.e., North America, Europe, and Australia (Schmull et al., 2005; Gallego and Sanchez, 2011; Thiers et al., 2016; Monfils et al., 2017; Cantrill, 2018; Haque et al., 2018; Kovtonyuk et al., 2018; Seregin et al., 2018; Brenskelle et al., 2019). This system has recently been implemented in many institutes in eastern Asiatic countries as well. In Korea, the National Institute of Biological Resources of Korea (https://species.nibr.go.kr/index.do) and the Korea Forest Service (http://www.nature.go.kr/main/Main.do) provide various types of information on the biodiversity of the country in addition to information about virtual herbaria. In China, a virtual herbarium that contains specimens deposited from various herbaria in China is available to the public from the Institute of Botany of the Chinese Academy of Science (https://www.cvh.ac.cn). In Taiwan, a database of the Herbarium of Academia Sinica, Taipei (HAST) with specimen images has been released (http://www.hast.biodiv.tw/Announce/newsE.aspx). These virtual herbaria have facilitated the sharing of information easily among researchers and have augmented traditional botanical research (Oh et al., 2010; Dodd et al., 2016; Shalimov et al., 2019; Aguilar-Cano and Hind, 2020).
Difficulties related to the limitation and restriction of specimen loans and travel to herbaria can be overcome by using information from virtual herbaria. During the COVID-19 (coronavirus disease 2019) pandemic, traveling to foreign herbaria was difficult. Specimen loans, though not prohibited, were not readily available due to higher shipping costs and understaffing issues. An analysis of the morphological variation of the D. kiusiana complex, which requires materials from China, Taiwan, Korea, and Japan, could be an excellent case study of the use of collections from various virtual herbaria.
Thus, aiming to provide objective evidence of the morphological and geographic variations of the D. kiusiana complex, we (1) investigated the morphological variations among the members of the complex, (2) evaluated the utility of digitalized herbarium specimens, and (3) clarified the taxonomic delimitation of the D. kiusiana complex.
MATERIALS AND METHODS
Herbarium specimens of the D. kiusiana complex were examined from CNU, KB, TNS, and TUT (herbaria acronyms according to Thiers, 2022). We also investigated the virtual herbaria of HAST and PE, which provide high-resolution specimen images of Taiwanese and Chinese collections, respectively. From these materials, we selected 81 specimens that were morphologically complete and suitable for morphometric measurements (Appendix 1). For an evaluation of the morphological variation within and among the populations, we included multiple specimens. Thirty-three specimens for D. kiusiana var. kiusiana were selected from accessions from Geojedo Island (17 specimens), Bigeumdo Island (6), and Japan (10). Each of the Japanese specimens represented a different population, but in this study, we regarded them as one large population. The Korean populations are all restricted to offshore islands and are isolated from the Japanese population. Thus, we included as many specimens from the islands as possible in the morphometric analysis to evaluate the range of morphological variation in Korea. Thirty-one specimens for D. kiusiana var. atrocaulis (14 from mainland China and 17 from Taiwan) were included. For D. jejudoensis, 17 specimens were investigated. Each specimen was treated as an operational taxonomic unit (OTU) for the morphometric analysis.
Twelve morphological characters (one qualitative and 11 quantitative) (Fig. 2, Table 1) were analyzed for each specimen that had fully mature leaves and flowers. As the leaf shape has been used to distinguish each taxon of the D. kiusiana complex (Lee et al., 2013), seven quantitative traits of shape parameters (C1–C7) (Table 1) were analyzed. Traits representing floral size (C8, C9, C11, and C12) were also included. Pubescence of the outer surface of the hypanthium was treated as a qualitative trait and scored as 0 (glabrous) or 1 (pubescent). The density of the trichome was not considered because there was no gap between dense pubescence and sparse pubescence.
The image analysis program ImageJ, which is freely downloadable from the ImageJ website (https://imagej.nih.gov/ij/), was used to measure the characters from the specimen images. The measurement unit was calibrated in the metric system with the scale provided in each image. To make the measurements consistent, specimens of CNU, KB, TNS, and TUT were digitized on an actual scale before the morphometric measurements.
A data matrix which consists of 81 OTU x 12 characters was constructed and used in the univariate and multivariate analyses (Appendix 2). In the univariate analysis, the range of variation and the mean of eleven quantitative characters were calculated for three groups in var. kiusiana (Geojedo Island, Bigeumdo Island, and Japan) as well as var. atrocaulis and D. jejudoensis. All statistical analyses including the principal component analysis (PCA) were conducted using the IBM SPSS program version 25 (IBM Corp., Armonk, NY, USA).
RESULTS
The variation patterns of the morphological characters measured here are shown in Fig. 3, and the statistics of the traits are provided in Table 2. In general, the quantitative characters show a wide range of variation within populations or geographic regions (Fig. 3; Table 2). It is difficult to find gaps by which to recognize separate species or varieties (Fig. 3; Table 2). Characters that may represent the leaf shape (C1–C7; see Table 1 for definitions of character abbreviations) also show a range of variations overlapping across taxa (Fig. 3). When the mean values were considered, a few characters exhibited differences among populations or taxa. The mean of C3 in Geojedo is slightly larger than those in other populations. The mean of the angle of the leaf apex (C7) in Geojedo was largest among the five populations, and that of var. atrocaulis was smallest, though wide ranges of variation were found within the populations. The hypanthium length (C8) of D. jejudoensis is longer than those in D. kiusiana (Fig. 3). Likewise, the sepal length (C11) of D. jejudoensis is longer than those in D. kiusiana (Fig. 3). The length of the hypanthium tends to correlate with the sepal length and width (Fig. 3, Table 2). However, these characters (C8, C11, and C12) varied considerably within D. kiusiana. The mean of the Japanese population (var. kiusiana) was lowest, followed by the mean of var. atrocaulis. The qualitative character (C10) was the only trait that distinguished D. jejudoensis from D. kiusiana.

Maximum, minimum, and mean values for the characters analyzed in this study. Horizontal glyphs represent the mean. Character numbers correspond to those in Table 1. A–C: D. kiusiana var. kiusiana (A=Geojedo, B=Bigeumdo, C=Japan); D. D. kiusiana var. atrocaulis (China and Taiwan); E: D. jejudoensis (Jejudo).
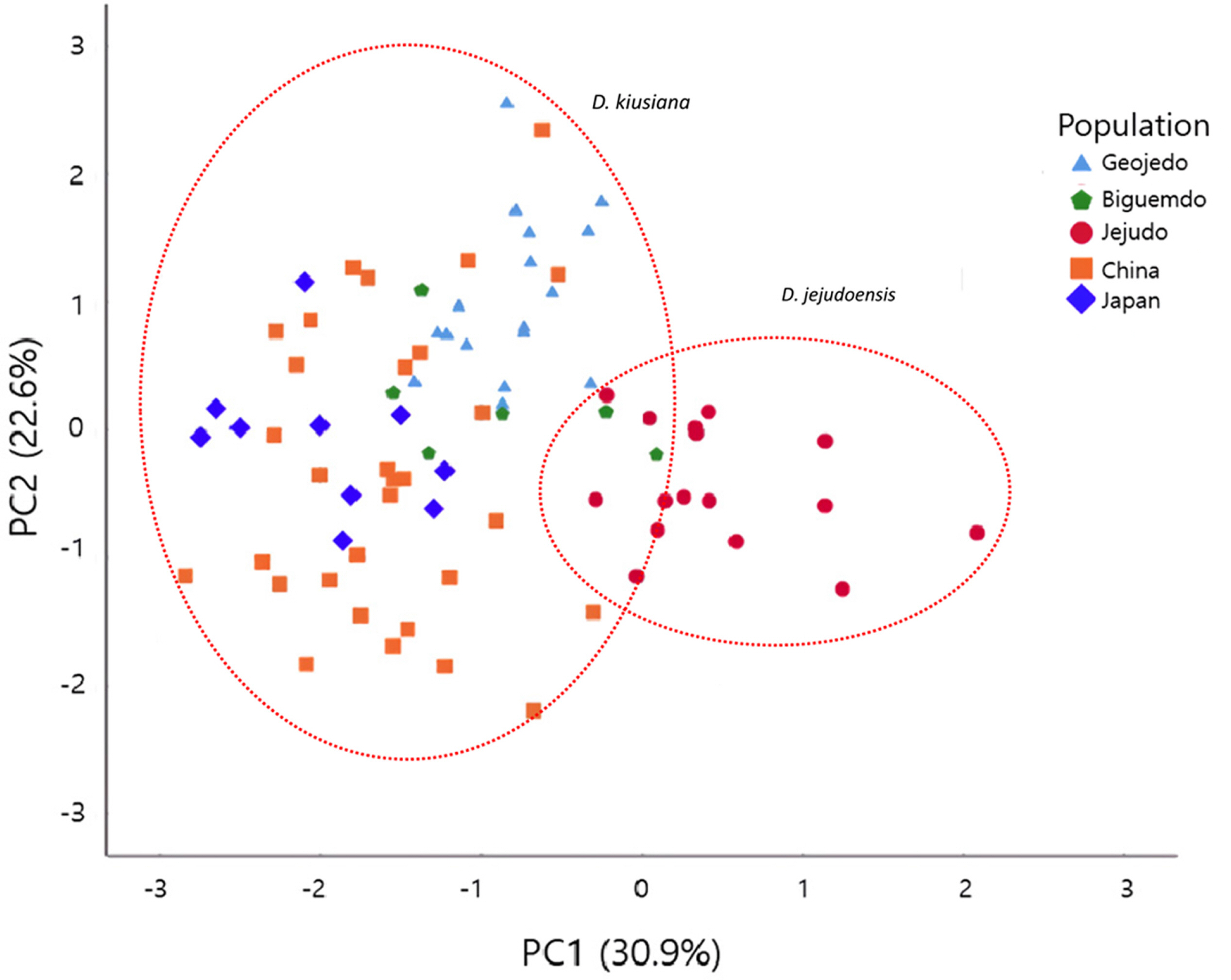
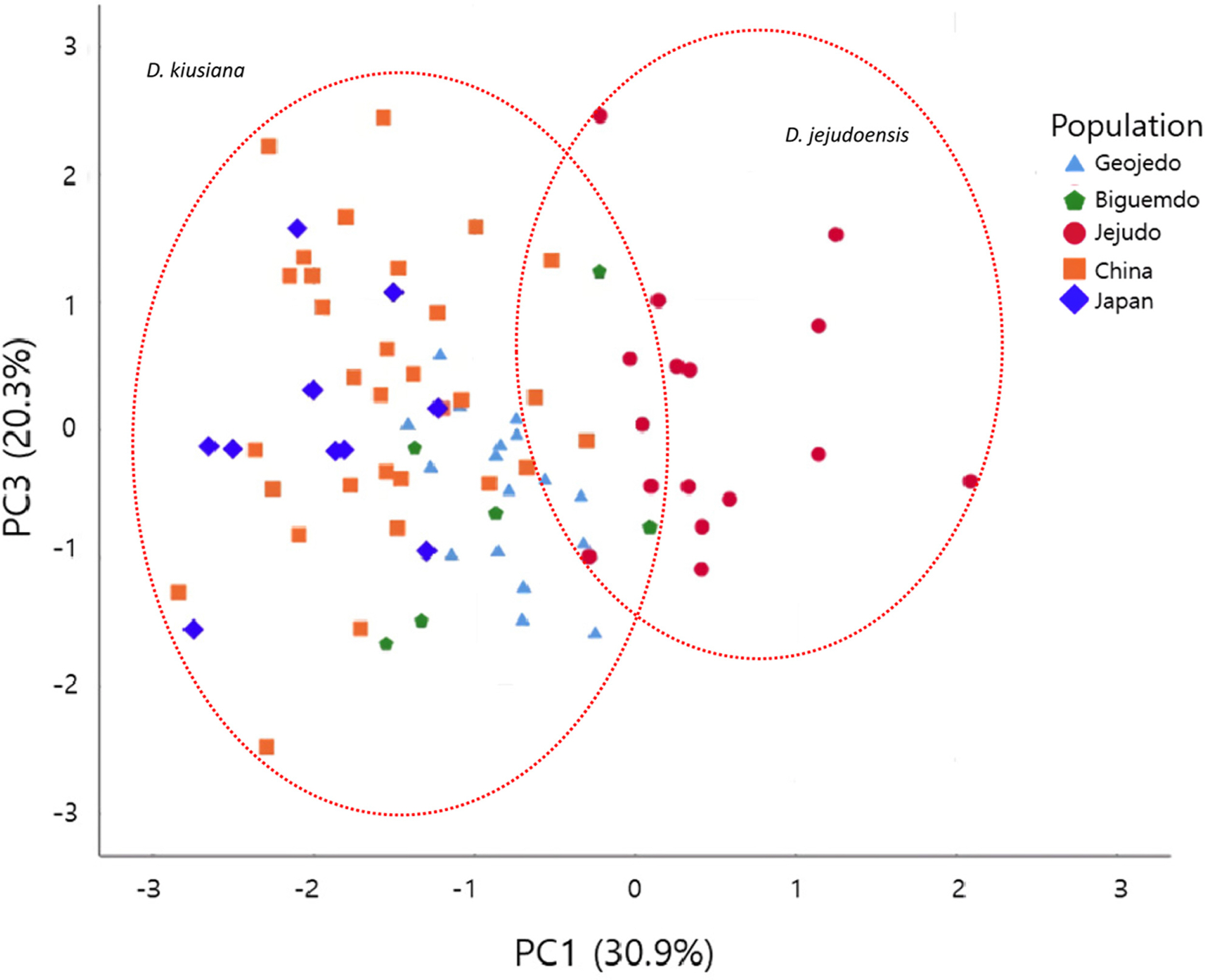
The PCA results revealed that the first three principal components (PC1, PC2, and PC3) account for 73.8% of the total variation. PC1, PC2, and PC3 explain 30.9%, 22.6%, and 20.3% of the variation, respectively (Table 3). Characters heavily loaded in PC1 were all floral traits (C8–C12) and those heavily loaded in PC2 and PC3 were leaf shape parameters (Table 3). Traits corresponding to the leaf width (C2, C3, and C6) and leaf apex angle (C7) strongly contributed to PC2, while characters of the leaf length (C1) and distance from the base to the point of maximum width (C4) were loaded heavily in PC3 (Table 3).

Loading of the first three principal components for 12 characters from the analysis of 81 individuals of D. kiusiana and D. jejudoensis
A scatter plot of the first two principal components showed that most individuals of D. jejudoensis were located on the positive axis of PC1, weakly separated from most individuals of D. kiusiana (Fig. 4). This trend is consistent with the univariate analysis (Fig. 3, Table 2), in which the hypanthium and sepals of D. jejudoensis are larger, on average, than those of D. kiusiana. However, two plants from Bigeumdo were nested within a group that consisted of individuals of D. jejudoensis. Three plants from Geojedo and one from China overlap in the D. jejudoensis group. The Japanese individuals tend to be on the negative axis, consistent with the results of the univariate analysis (Fig. 3; Table 2), in which the means of the characters representing the flower size were small (C8, C9, C11, and C12). There were no significant patterns between the Chinese and Taiwanese plants, which showed a wide range of variation across the PC1 axis.
PC2 did not place the OTUs into any taxonomic group or any discernible geographic grouping. The individuals of Geojedo tend to be located on the positive axis, while those of Jejudo (D. jejudoensis) are on the negative axis (Fig. 4).
A scatter plot of PC1 and PC3 (Fig. 5) showed patterns similar to the plot of PC1 and PC2 (Fig. 3). Individuals of D. kiusiana and D. jejudoensis show a wide range of variation along PC3, resulting in no clear groupings. PC2 and PC3 are highly correlated in terms of their leaf characters (C1–C4, C6, and C7), as stated previously, and the leaf characters (size and shape) may not be useful taxonomically in the D. kiusiana complex.
DISCUSSION
Daphne jejudoensis is a recently recognized species from Jejudo based on elliptic leaves with an acuminate apex, a glabrous hypanthium, and a longer hypanthium and sepals (Lee et al., 2013). PCA results here show that the OTUs of D. jejudoensis tend to be separate from those of D. kiusiana by PC1 (Figs. 4, 5), which is correlated with the size of the hypanthium (C8, C9) and sepals (C11, C12), as well as the pubescence of the hypanthium (C10). However, D. kiusiana and D. jejudoensis are not clearly separable. Six individuals of D. kiusiana from Bigeumdo, Geogedo, and China overlap with D. jejudoensis (Figs. 4, 5). This pattern suggests that the floral characteristics found in those individuals of D. kiusiana may be similar to those in D. jejudoensis. Some plants of D. kiusiana var. kiusiana on Bigeumdo, such as accession numbers 7878 and 7879 (Appendix 2), show relatively long hypanthia and sepals (Fig. 3), not clearly distinguished from D. jejudoensis. All of the materials of D. jejudoensis included in our morphometric analysis show a glabrous hypanthium (Fig. 3). However, individuals with a pubescent hypanthium were occasionally found in the investigation of the specimens in D. jejudoensis, suggesting that this qualitative characteristic may be incompletely fixed within the Jejudo population.
Leaf shape is not an accurate indicator to support D. jejudoensis. Variables that represent the leaf shape (C3–C6) and apex (C7) are loaded in PC2, which does not have the resolution to separate D. jejudoensis from D. kiusiana. Our examination of specimens indicates that the leaf shape is highly variable in the D. kiusiana complex, in which elliptic, elliptic-oblong, lanceolate, and oblanceolate leaves are found. It appears that the leaves of Geojedo and Japan are oblanceolate, with the widest point located above the middle of the leaf blade (Ohwi, 1965; Murata, 1999). The character C5 quantifies the relative position of the widest point of a leaf blade (Fig. 2, Table 1). Our measurements indicate that the means and ranges of the Geojedo and Japan populations are similar to those of other populations (Fig. 2; Table 2). Plants on Geojedo tend to have an acute apex, but this varies within the population, and specimens of other populations show a wide range of variation from an acute to an acuminate apex.
Lee et al. (2013) also highlighted that D. jejudoensis is distributed in forests in the interior areas of Jejudo, whereas D. kiusiana occurs in the coastal region. This may be valid when only the specimens of Korea are considered. As plants in Japan, China, and Taiwan are distributed in forests in various regions at low elevations based on information on the specimen labels examined in this study and from the literature (Ohwi, 1965; Wang et al., 2007), there is no differentiated habitat preference among the taxa.
Daphne kiusiana var. atrocaulis was established as a new variety based on a collection in eastern Sichuan, China, and is distinguished from var. kiusiana by having caducous bracts, thicker leaves, and dark purple branchlets (Rehder, 1916). Since then, plants distributed in China and Taiwan have been classified in var. atrocaulis (Li, 1977; Wang et al., 2007). However, our examination of specimens of the D. kiusiana complex shows that these characteristics do not have diagnostic value. Bracts of var. kiusiana are caducous, as in var. atrocaulis. The color of the branchlets also varies, ranging from light brown to dark purple in specimens of the D. kiusiana complex. The holotype of var. atrocaulis (Henry 7119 housed in GH) is a sterile material and has persistent leaves developed in previous years. The material shows that the youngest branchlet is darker in color tone than branches developed in previous years, suggesting that the type material may have had a dark purple branchlet. The thickness of the leaves is a complex character to be determined in herbarium specimens.
Wang et al. (2007) noted that var. kiusiana differs from var. atrocaulis by oblanceolate leaf blades and smaller flowers with the hypanthium 7–8 mm long. Our morphometric analysis shows that the hypanthium of var. atrocaulis is not significantly longer than that of var. kiusiana (Fig. 3, C8). As discussed previously, the leaf shape in var. kiusiana is variable.
Thus, our morphometric analysis suggests that recognition of var. atrocaulis is not supported. Recognition of D. jejudoensis as a distinct species needs further investigations using molecular data. Our morphometric analysis, which shows that D. jejudoensis is not clearly separable (Figs. 3–5), suggests the possibility of the recognition of D. jejudoensis as a variety of D. kiusiana. The D. kiusiana complex is distributed in lowlands of warm temperate regions of eastern Asia, referring to areas that have been connected and disconnected during the last glaciation and deglaciation period over the past 20,000 years. It is likely that current populations of the D. kiusiana complex are fragmented and isolated across the region. The flowers of Daphne, including those of the D. kiusiana complex, have a nectary disk at the base of the carpel, and the fruits when mature are bright red in color, implying that animals may be involved in pollination and dispersal (Oh and Hong, 2015). Further studies are needed to reveal the genetic diversity within and among populations and the relationships and structures of the populations of the D. kiusiana complex.
Our morphometric analysis showed that digitized images of herbarium specimens can be useful for morphological analyses (Figs. 3–5). An image analysis program like the one used in this study, ImageJ developed as an open-resource project (https://imagej.net), can be a valuable tool for handling digitized herbarium specimens. Traditionally, morphological characters are usually scored based on an examination of actual specimens (Lee and Park, 1994; Lee et al., 2021; Islas-Hernández et al., 2022). In most cases, researchers borrow specimens from herbaria to examine and measure the characters or visit herbaria to obtain morphological data. Recently, digital images of herbarium specimens and associated information have become available on herbarium websites (Barkworth and Murrell, 2012). Although limitations exist with regard to the complete replacement of actual specimens, as some characters, particularly those of anatomical features, are difficult to examine, the use of a virtual herbarium provides an additional method for investigating more diverse specimens and taxa.
Acknowledgements
We are grateful to Jung-Hyun Lee for providing the Japanese materials and to Tomohisa Yukawa for allowing the examination of herbarium specimens at the Tsukuba Botanical Garden, National Museum of Nature and Science (TNS), Kuo-Fang Chung of Academia Sinica, Taiwan (HAST), and staff of the herbarium of vascular plants of the National Institute of Biological Resources (KB). We thank Hwa-Jung Suh, Su-Chang Yoo, and Yun-Kyeong Choi for their help throughout the project, and Dong-Hyuk Lee for the photograph of D. kiusiana from Bigeumdo. We are grateful to two anonymous reviewers for their invaluable comments and suggestions. This paper represents part of an MS thesis submitted by the first author in partial fulfillment of the requirements of an MS degree from Daejeon University. This work was supported by research grants from the National Institute of Biological Resources of Korea (NIBR-202005201).
Notes
CONFLICTS OF INTEREST
Sang-Hun OH, the Editor-in-Chief of the Korean Journal of Plant Taxonomy, was not involved in the editorial evaluation or decision to publish this article. The authors have declared no conflicts of interest.
References
Appendices
Appendix 1. Specimens of the Daphne kiusiana complex analyzed for the morphometric analysis
D. jejudoensis M. Kim: KOREA. Jeju-do: Jeju-si, Hangyeong-myeon, Cheongsu-ri, Cheongusugotjawal, 13 Feb 2020, S. H. Oh & H. J. Suh 7843, 7844,7845, 7846, 7847, 7848, 7849, 7850, 7851 (TUT); Jeju-si, Hangyeong-myeon, Cheongsu-ri, Cheongusugotjawal, 30 Mar 2020, S. H. Oh 7883 (TUT); Jeju-si, Hangyeong-myeon, Jeoji-ri, Jeojigotjawal, 13 Feb 2020, S. H. Oh & H. J. Suh 7852, 7853, 7854, 7855 (TUT); Jeju-si, Hangyeong-myeon, Jeoji-ri, Jeojigotjawal, 13 Feb 2020, D. H. Lee 7882 (TUT); Jeju-si, Hangyeong-myeon, Cheongsu-ri, Sanyanggotjawal, 27 Mar 2014, G. H. Nam et al. 467671 (KB); Jeju-si, Jocheon-eup, Seonheul-ri, Seonheulgotjawal, 25 Mar 2013, M. H. Kwak et al. 428151 (KB); Jeju-si, Jocheon-eup, Seonheul-ri, Seonheulgotjawal, 2 Mar 2018, G. P. Song et al. 718968 (KB).
D. kiusiana Miq. var. kiusiana: KOREA. Gyeongsangnam-do: Geoje-si, Dapo-ri, 20 Feb 2020, S. H. Oh et al. 7862, 7863, 7864, 7865, 7866, 7867, 7868, 7869, 7870, 7871 (TUT); Geoje-si, Geogu-ri, 20 Feb 2020, S. H. Oh et al. 7856, 7857, 7858, 7859, 7860, 7861 (TUT). Jeollanam-do: Sinan-gun, Bigeumdo, 14 Mar 2020, D. H. Lee, 7875, 7876, 7877, 7878, 7879, 7880 (TUT).
JAPAN. Honshu: Chiba Pref.: Awapgun, Amatsu, Todaichiba, 23 Mar 1968, S. Uehara 223542 (TNS); Koutou, Kiyosumi, 27 Mar 1963, T. Wakana 155810 (TNS); 27 Feb 1966, H. Georges 996574 (TNS). Hyogo Pref.: Tanba-gun, Sasagamine, 26 Apr 1936, S. Hosomi 56748 (TNS). Mie Pref.: Watarai-gun, Kajiya-dani Valley, 4 Apr 1996, Fujii et al. 678564 (TNS). Tottori Pref.: Iwami-gun, 5 Apr 1975, K. Iwatsuki et al. 335713 (TNS). Yamaguchi Pref.: Nagato, Abu-gun, Kasekajaka, 18 Mar 1919, J. Nikai 47295 (TNS). Shikoku: Kagawa Pref.: Shodoshima, Kankakei, 28 Mar 1962, N. Soromu 19573 (TNS). Kyushu: Miyazaki Pref.: Kobayshi, 8 Jan 2019, J. H. Lee. 8238 (CNU); Noveokashi, 9 Jan 2019, J. H. Lee. 8239 (CNU).
D. kiusiana var. atrocaulis (Rehder) F. Maek.: CHINA. Fujian: Fuzhou, 1943, L. Yong 5475 (KW 02135789); Fuzhou, Nanping, 22 Nov 1981, K. W. Heo 1610 (PE 1270226). Guangdong: 11 Dec 1957, J. Huang 44396 (PE 724105); 2 Dec 1978, H. F. Chow 13764-1(KW 227068); Lecheng, 22 Dec 1936, L. Yao 2185 (KW 515031). Guangxi: Zhuangzu, 24 Nov 1978, Chow 358709 (PE). Hunnan: 30 Jan 1996, without collector’s name (PE 1469159); Zhanguha, 10 Nov 1975, Manchowae 1166492 (PE). Zhejjang: 15 Oct 1927, Chun 02135791, 227054, 227055 (KW); Hangzhou 18 Mar 1957, Zhang 0552192 (PE); Hangzhou 25 Feb 1963, Yang 0945737 (PE). Without specific locality, 11 Feb 1993, Lin et al. 28150 (HAST).
TAIWAN. Chilan: 7 Jan 1996, Lu 59337 (HAST). Hualien: 29 Nov 2008, Lu 123919 (HAST). Ilan: Mingchih, 30 Jan 1996, Lu 69614 (HAST). Nantou: 15 Jan 1993, Chen 24227 (HAST); 23 Feb 2002, Wang & Lin 2345927 (PE). Shihlu; 19 Feb 2002, Huang 90323 (HAST); 21 Feb 2002, Huang 89200 (HAST). Taipei: 22 Feb 1987, Ryu 16443 (HAST); 7 Mar 1996, Kuo 70168 (HAST); 15 Mar 1997, Chiang 69995 (HAST); Mt. Yangming, 9 Feb 1987, Peng et al. 8845 (HAST); 24 Feb 1997, Kuo 70143 (HAST); 24 Feb 2005, Huang 112788 (HAST). Yaoyuan: 4 Jan 1996, Wang 64360 (HAST). Without specific locality, 15 Jun 2015, Liang 245233, 2455343, 2455344 (HAST).