An overlooked invasive alien plant of Jejudo Island: Commelina caroliniana (Commelinaceae)
Article information
Abstract
Invasive alien species management is pivotal for biodiversity conservation. Commelina caroliniana Walter, from the family Commelinaceae, is an alien plant native to the Himalayas and India, but it has been widely introduced around the world, including in the United States, Brazil, Philippines, and Japan. In Korea, the first population was found growing adjacent to agricultural land and farm roads on Jejudo Island, and field observations confirmed the presence of at least nine populations there. It is similar morphologically to C. diffusa Burm. f. but can be distinguished by involucral bracts that are ciliate at the base, hairs on the peduncle and obsolete upper cincinnus, brown spots on its 4-lobed antherode, and seed surfaces that are smooth to slightly alveolate. It was determined to have an invasiveness low score of 8 according to the Korean ‘Invasive Alien Plant Risk Assessment’, suggesting that it may spread to natural habitats. Although the current distribution of C. caroliniana is restricted to Jeju-si, it has spread dramatically in many other areas of the world. At present, it has had a limited impact on the local environment, but local and regulatory authorities should pay close attention to this plant and take measures to prevent its expansion in the future.
Commelinaceae Mirb. is a relatively large family, containing 41 genera and 650 species worldwide (Hong, 2000; Lee, 2006; Gajurel and Shrestha, 2009; Fukuoka and Iwatsuki, 2016; Ezeabara et al., 2019). The family is morphologically characterized by a prominent sheath or involucral bracts with a leaf blade containing moisture, and a flower split into three parts (Evans et al., 2000; Hong, 2000; Fukuoka and Iwatsuki, 2016).
Commelinaceae was initially classified into two families, Commelineae and Tradescantieae, by Meisner (1842). Later, Faden and Hunt (1991) divided Commelinaceae into two subfamilies, Cartonematoideae and Commelinoideae, and included two tribes, Commelineae and Tradescantieae, within Commelinoideae. Additionally, Evans et al. (2000) used a molecular approach to show the cladogram for the Commelinaceae family.
In the family Commelinaceae, the largest genus is Commelina L., which consists of approximately 170 species (Kaul and Koul, 2012; Nandikar and Gurav, 2018) and it is particularly diverse in Africa where there are 120 species (Faden, 2012). However, the identity of some species is unclear due to nomenclatural and taxonomic problems such as the absence of a type specimen and lack of detailed morphological descriptions in the protologue (Faden, 1989). Furthermore, Commelina is an increasingly confusing genus, as new species are continuously described without taxonomic verification based on the characteristics of the vegetative organs or the physiology of previously accepted taxa (Hassemer, 2019). In Korea, C. benghalensis L. and C. diffusa Burm.f. (Kim and Kim, 2011) have recently been identified as alien species. There are five taxa (Lee, 2003; Lee, 2006; Lee, 2007; Korea National Arboretum, 2017, 2019; Lee, 2018), known to occur in various habitats (Hong, 2000; Fukuoka and Iwatsuki, 2016; Lee, 2018).
During a recent field survey of alien plants on Jejudo Island, we discovered an unusual and rather isolated population of Commelina in Jongdal-ri of Jeju-si (Fig. 1), which appeared to be morphologically differentiated from the other Commelina taxa previously recorded in the flora of Korea (Lee, 2003; Lee, 2006; Lee, 2007; Kim and Kim, 2011; Lee, 2018). After examining various floras and herbarium specimens from Korea and adjacent countries as well as the relevant literature (Faden, 1989, 1993, 2000; Hong, 2000; Lee, 2003; Lee, 2006; Kaul et al., 2007; Lee, 2007; Kim and Kim, 2011; Nampy et al., 2013; Joseph and Nampy, 2015; Fukuoka and Iwatsuki, 2016; Korea National Arboretum, 2017; Lee, 2018; Nandikar and Naik, 2019), it was concluded that the collected specimens were C. caroliniana Walter, a species native to Himalaya and India that has been introduced into many areas of the world, including the United States, Brazil, Philippines, Guyana, and Japan. Here, we have provided a detailed description, and color photographs of this newly recorded invasive alien plant in the flora of Korea, and this information could be utilized to help effectively manage it.

Commelina caroliniana Walter, which was found in Jongdal-ri, Jeju-si, Jeju-do. A. Collection site of Commelina spp. B. Habitat of C. caroliniana, which was first found in October, 2019. C. Commelina caroliniana spreading along the agricultural land and farm road. D. Commelina caroliniana population forming dense mats.
Materials and Methods
Morphological observations of the new alien species were conducted on living plants and herbarium specimens in 2019 and 2020. The field photographs were captured with a Nikon Coolpix P510 camera (Tokyo, Japan). The morphological characteristics were measured using a Mitutoyo 500-196-30 absolute digimatic Vernier caliper (Kanagawa, Japan), and the data were derived from the field notes. The flowering and fruiting periods were given as cited on the collector’s labels. The examined material has been deposited in the Korea National Arboretum (KH). To confirm the identity of C. diffusa in Korea, we investigated the habitat (Yongdam-dong, Jeju-si), which was reported by Kim and Kim (2011) and voucher specimens of C. diffusa that were stored at the National Institute of Biological Resources (KB). The invasiveness status was assessed by applying the Invasive Alien Plant Risk Assessment system (Jung et al., 2015).
Results
Commelina caroliniana Walter, Fl. Carol. 68, 1788.—TYPE: U.S.A. South Carolina, Walter (or Fraser?) s.n., Walter Herbarium, p. 35, upper right-hand corner (lectotype, BM!, designated by Faden, 1989) (Fig. 2).

Photographs of C. caroliniana from Jejudo Island, Republic of Korea. A. Polygamous plant. B. Roots at nodes. C. Leaves. D. Ciliate at base of spathe-like involucral bracts. E. Upper cincinnus exerted from involucral bracts (pedicel of apical upper cincinnus is mostly obsolete and short, rarely with 1.5–2 cm long pedicel). F. Male flower. G. Bisexual flower. H. Staminode (brown spot at the center of antherodes in staminode). I. Capsules (right, posterior valve with 1-seed; left, 2 anterior valves each with 2-seeds). J. Seeds.
Commelina hasskarlii C.B.Clarke, Commelyn. Cyrtandr. Bengal. 13, t. 3, 1874.
Korean name: Nu-un-dak-ui-jang-pul (누운닭의장풀).
Herbs annual, diffusely spread. Rhizome absent; roots at the nodes. Stems decumbent to scandent, branched at basal, more than 1 m long, glabrous. Leaves alternate, sessile or subsessile; sheath hispid-ciliate, with green to red veins; blade lanceolate or lanceolate-oblong, 2.2–6.2 cm long, 0.7–1.5 cm wide, apex acute, margin entire, base cordate or rounded, light green, glabrous. Inflorescence cincinni included in involucral bract, dichotomously branched from base; upper cincinnus mostly obsolete and short, rarely with 1.5–2 cm long pedicel exerted from involucral bracts, male flower 1; other cincinnus with much shorter pedicel, bisexual flowers 1–2. Involucral bracts opposite to leaf, spathe-like, folded or slightly funnel-form, basal detached, 1.2–2.5 cm long, 1.25–1.7 cm wide, apex acuminate, entire to repand, abaxially glabrous or sparselypubescent, ciliate at base; peduncle pubescent. Flowers zygomorphic, andromonoecious; inner sepals 2, often connate at base, ovate-orbicular, 3–4 mm long, membranous; petals 3, free, blue, spatulate or orbicular, longer ones 2, 8–11 mm long, 7–10 mm wide, small one 1, 6–8 mm long, 4–5 mm wide; fertile stamens 3, longer ones 2, shorter one 1; filaments glabrous; staminodes 3, 4-lobed, butterflylike, antherodes developed; antherodes with brown spot or absent; ovary 3-loculed, posterior locule 1, ovule 1, anterior locules 2, each ovules 2. Fruit capsule, ellipsoid, 4–7 mm long, 5–6 mm wide, light green, 3-valved; posterior valve 1-seed, indehiscent; anterior valve 2, each 2 seeds, dehiscent. Seeds brown to dark brown, smooth to slightly alveolate surface; longer ones 4–4.8 mm long, 1.6–2 mm wide, oblong; small 2.8–3 mm long, 1.6–2 mm wide, ovate-spherical, truncate at base.
Phenology: August to September, usually begins to bloom after the rainy season.
Distribution: Bangladesh, Brazil, Guyana, India, Japan, Korea (Jejudo Island), Nepal, Philippines, United States.
Specimens examined: GUYANA. Georgetown, 3 Jun 1987, B. Boom, M. Blackman and R. Mohabir, 01392710 (NY).
KOREA. Jeju-do: Jeju-si, Jongdal-ri, 30 Oct 2019, KH1910301, KH1910302, KH1910303, KH1910304 (KH); 10 Sep 2020, KESUA0003, KESUA0004, KESUA0005, KESUA0006 (KH); Jeju-si, Yongdam 3(Sam)-dong, 14 Sep 2019, NIBRVP0000759501, NIBRVP0000759502 (KB); 5 Oct 2019, NIBRVP0000759503, NIBRVP0000759504 (KB); 10 Sep 2020, KESUA0001 (KH); Jeju-si, Hado-ri, 10 Sep 2020, KESUA0002 (KH); Seogwipo-si, Sagye-ri, 10 Oct 2020, KJS0691 (KH).
NEPAL: Dhankuta, 21 Oct 1963, H. Hara, H. Kanai, S. Kurosawa, G. Murata, M. Togashi and T. Tuyama, 10002301 (TI).
U.S.A. Florida: Jacksonville, 2 Oct 1893, A. H. Curtiss, 03733347, 03733348 (NY); Louisiana: New Orleans, 16 Oct 1989, Toby Feibelman, 03733334 (NY); Louisiana: Cameron, 18 Nov 1983, R. Dale Thomas, B. E. Dutton and D. D. Taylor, 03733350 (NY); Louisiana: Medison, 8 Nov 1983, R. Dale Thomas, 03733351 (NY).
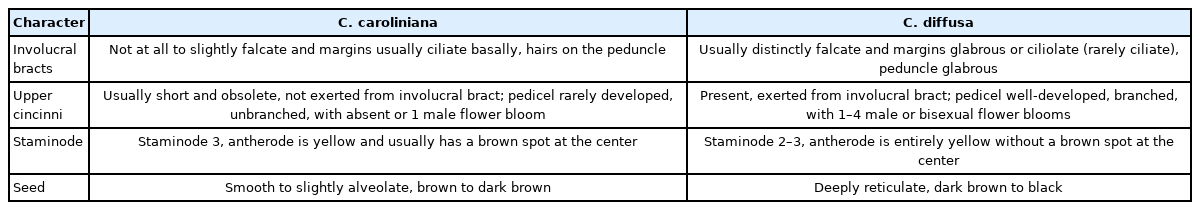
Taxonomic note: Commelina caroliniana is morphologically similar to C. diffusa by having creeping and diffusely spreading stems on the ground, acuminate apex in the involucral bracts (Fig. 2A, B), and the five seeds contained in the three-locular capsule (Fig. 2I) (Faden, 1989, 1993). Despite these similarities, there are clear differences between the two species, such as the shape of the involucral bracts, the trichomes of the involucral bracts and peduncle, the number of flowers on the upper cincinni, the presence of a brown spot on the central part of the antherode in the staminodes, the surfaces and color of the seeds, as well as the chromosome numbers (2n = 30 for C. diffusa vs. 2n = 86 for C. caroliniana) (Faden, 1989, 1993; Nandikar and Naik, 2019) (Table 1, Fig. 2).
Habitat and ecology
Based on field observations, at least nine populations of this species were observed on Jejudo Island, Korea. They either formed dense mono-species stands or stands mixed with native plants, including C. communis L., Phyllanthus urinaria L., Acalypha australis L., Humulus scandens (Lour.) Merr., Euphorbia humifusa Willd. ex Schltdl., Lactuca indica L., and alien plants, Erigeron floribundus (Kunth) Sch. Bip., and Veronica persica Poir. in a herbaceous layer.
Invasiveness risk assessment in forests
Commelina caroliniana originates in Himalaya and India and has recently been identified as an invasive alien plant in the United States and Japan. In the study, C. caroliniana was assigned a slightly low invasiveness score of 8, suggesting that the potential invasion is minor in the forest, according to the classification by Jung et al. (2015). It is a casual alien plant mainly distributed in agricultural land and lowland farm roads, forming dense mats. Each population identified included over 200 mature individuals that seemed to have been naturalized plants as they formed a sustainable population growing naturally with the native plants in herbaceous layers. It is difficult to trace how this species was introduced to Korea, but considering the environment of the habitat, we can assume that it was unintentionally introduced after being mixed with rice or other crops planted in the fields. Although the current nine habitats identified for C. caroliniana were restricted to agricultural land and farm roads in Jeju-do Province, these areas are prone to invasion of alien plant species owing to their frequent exposure to stress and disturbance (Kim et al., 2019); thus, as this species has a strong ability to spread, it may cause difficulties in the future for agricultural land management. Furthermore, there is evidence that it has spread dramatically in many other areas of the world. Therefore, local and regulatory authorities should pay close attention to this plant and take measures to stop its expansion.
Key to the species of Commelina in Korea
1. Proximal margin of involucral bracts connate ···················· ······································································· C. benghalensis
1. Proximal margin of involucral bracts open or folded but not basally connate, rounded.
2. Involucral bracts funnel-formed; locules of capsule 3, seeds 5 ······················································ C. caroliniana
2. Involucral bracts cardioid-shaped; locules of capsule 2, seeds 4
3. Flower less than 1 cm in diam. ················· C. minor
3. Flower more than 1 cm in diam.
4. Leaves broadly lanceolate; proximal petal white · ·································· C. communis var. communis
4. Leaves lanceolate; proximal petal light blue ······· ······························· C. communis var. angustifolia
Discussion
Commelina caroliniana is native to Himalaya and India, but was first reported in South Carolina, United States, where it was known to be introduced during the importation of grain in 1696 (Rehel, 2013). Currently, the original habitat of C. caroliniana is gradually disappearing due to urban development, whereas in the United States, it is widely naturalized in the southeast and has been designated as an invasive alien plant that damages crops (Faden, 1993; Rehel, 2013; Kraus et al., 2020). Commelina caroliniana is also naturalized in Japan, and it is speculated that it was introduced to Kyushu during the importation of rice from India (Matsuo et al., 2016).
Previously, the identity of C. caroliniana was unclear and overlooked, and it was treated as a synonym of C. diffusa (Clarke, 1881; Britton and Brown, 1896; Pennell, 1938; Brashier, 1966; Duncan and Kartesz, 1981) as they are morphologically very similar, and C. diffusa is more widely distributed in the world than C. caroliniana. However, Faden (1989, 1993) detected them to be different species by distinguishing differences in their external morphologies such as their involucral bracts, cincinni, staminodes, seeds, and the number of chromosomes (see Table 1).
Commelina diffusa was recently reported as an unrecorded alien species by Kim and Kim (2011) and was confirmed as being misidentified as C. caroliniana. In the images of C. diffusa presented by Kim and Kim (2011), there were hairs on the peduncle and ciliate of the basal involucral bracts, which are major characteristics of C. caroliniana. Not only that, but the seed had a slightly uneven surface, it was almost smooth, unlike the C. diffusa presented by Faden (1993). Furthermore, all four specimens of C. diffusa deposited at KB (NIBRVP0000759501, NIBRVP0000759502, NIBRVP0000759503, NIBRVP0000759504) have hairs on the peduncle and cilia at the basal involucral bracts (Fig. 3B), and the upper cincinnus in the involucral bracts are obsolete and short (Fig. 3E). Moreover, two sheets (NIBRVP0000759501, NIBRVP0000759502) which had flowers (Figs. 3A, D), displayed brown spots on the center of the antherode, which were not observed in C. diffusa (Figs. 3C, E). When we carried out investigations in Yongdam-dong, Jejudo Island, where C. diffusa was first found in Korea, C. diffusa could not be identified, but C. caroliniana along with C. communis were (Fig. 4). Therefore, we concluded that Kim and Kim (2011) potentially misidentified C. caroliniana as C. diffusa, and that C. diffusa is not distributed in Korea.

Voucher specimens, which are registered as C. diffusa at the National Institute of Biological Resources (KB). A. NIBRVP0000759502 B. Ciliate at base of involucral bracts and hairs on peduncle. C. Brown spot at the center of the antherode in the staminode. D. NIBRVP0000759501 E. Obscure pedicel at upper cincinnus and brown spot at the center of the antherode in the staminode.

Distribution map of Commelina caroliniana Walter from Jejudo Island, Korea. Triangle identifies the location, reported by Kim and Kim (2011) to contain C. diffusa Burm. f.
Meanwhile, it is presumed that C. caroliniana in Jejudo Island was unintentionally introduced after being mixed with rice or other crops planted in the fields, as occurred previously in the United States and Japan. This species seems to have been naturalized for a long time ago as it was reported in 2011 by Kim and Kim (2011) as C. diffusa, and currently, C. caroliniana is extensively distributed in Jejudo Island (Fig. 4).
In Korea, ecosystem damage caused by C. caroliniana has not yet been reported, but the possibility that C. caroliniana can spread to the surrounding farmland or natural ecosystem and cause damage cannot be completely neglected, as seen in the United States. Therefore, it is necessary to identify the possibility of spread and ecological impact, conduct periodic distribution surveys, and prepare measures to mitigate damage if necessary.
Acknowledgements
This study was supported by the Korea National Arboretum, project No. KNA1-2-39, 21-2.
Notes
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
Appendices
Appendix 1. Observed specimens Commelina diffusa Burm.f. in this study
ANTIGUA and BARBUDA. Gunthorpes, 22 Oct 1937, H. E. Box, 01503967 (NY).
BRAZIL. Sergipe: Maruim, 22 May 2013, L. A. Gomes, 02103608 (NY).
CHINA. Maguan: Gulinqing, 14 Oct 2002, Y. M. Shui W. H. Chen and J. S. Sheng, 31282 (PE); Yunnan: Luchun, 8 Aug 2006, Y. Shui and W. Chen, 70646 (PE).
CUBA. Pinar del Río: Punta Brava, 15 Nov 1904, C. F. Baker, 01503946 (NY); Havana: Santiago de Chile, 13 Nov 1904, H. A. van Hermann, 01503924 (NY).
U.S.A. Florida: Alachua, 26 Oct 1976, D. W. Hall, 165615 (FLAS); Brevard, 20 Mar 2002, J. E. Tear, 231820 (FLAS); Alabama: Tallapoosa, 18 Oct 2006, T. W. Barger and D. Tenaglia, 00014810 (AMAL); Monroe, 11 Sep 2012, T. W. Barger and B. D. Holt, 00019555 (AMAL); Alabama: Montgomery, 20 Oct 2011, A. R. Diamond, 0010106 (UWAL).
VIRGIN ISLANDS. Saint Croix: Frederiksted, 24 Jan 1980, F. R. Fosberg, 01503969 (NY).