Exploring natural hybridizations among Asplenium ruprechtii and related taxa in Korea
Article information
Abstract
The purported four hybrid origins of Asplenium in Korea were tested based on morphological, cytological and DNA sequence data. Asplenium castaneo-viride, A. × uiryeongse, A. × montanus, and A. × kitazawae share several morphological characteristics with the Asian walking fern A. ruprechtii and related taxa as parents and show a sympatric distribution with the putative parents, raising the possibility of hybrid origins: A. castaneo-viride (A. ruprechtii and A. incisum), A. × uiryeongse (A. ruprechtii and A. pekinense), A. × montanus (A. ruprechtii, A. trichomanes, and A. incisum), and A. × kitazawae (A. ruprechtii and A. sarelii). We investigated flow cytometry and chloroplast DNA sequence data (rbcL, rps4-trnS, and rps4-trnS intergenic spacer) to clarify the hybridization and origin of each hybrid. In the flow cytometry analyses, A. ruprechtii shows diploid (2x) only, whereas A. castaneo-viride (3x, 4x), A. × uiryeongse (3x), A. × montanus (3x, 4x), and A. × kitazawae (2x, 4x) exhibit polyploidy, suggesting hybrid events along speciation. The rbcL and rps4-trnS and rps4-trnS intergenic spacer data suggest that A. ruprechtii is one the maternal ancestors of all four hybrids. In addition, the rps4-trnS and rps4-trnS intergenic spacer data indicate that A. incisum is also the maternal ancestor of A. × kitazawae and A. × montanus, proposing multiple hybridization events for these two hybrids. In A. × montanus, morphological features such as the leaf forms and sympatric distributions of the species also support the multi-maternal hypothesis, but the morphological features of A. × kitazawae must be examined with consideration of hybrid events. To clarify the complex hybrid evolutionary lineages of the four Asplenium hybrids, further research with taxon sampling and molecular markers should be conducted.
The genus Asplenium L. (Aspleniaceae) is widely distributed throughout the temperate and tropical regions of all the continents except Antarctica, and comprises of approximately 700 species worldwide (Schneider et al., 2004a; Chang et al., 2013). Distinguishing characteristics of the genus are erect or shortly creeping rhizomes, dense scales throughout or on stipe bases, clustered or remote fronds, simple to 4-pinnate lamina and costa usually with all basal basiscopic veins, linear indusia, echinate spores, and n = 36 (Murakami and Schaal, 1994; Iwatsuki, 1995; Lin and Viane, 2012), especially compared with its related genus, Hymenasplenium (Murakami and Schaal, 1994; Hasebe et al., 1995; Murakami et al., 1999; Schneider et al., 2004b; Perrie and Brownsey, 2005). Fifteen to twenty taxa of Asplenium are known in Korea (Park, 1975; T. B. Lee, 1980; Y. N. Lee, 2006; Kim and Sun, 2007; Lee and Lee, 2015, 2018). Among them, autopolyploid and allopolyploid in Asplenium are common and have been investigated on the possibility of hybrid speciation in some taxa (Rumsey et al., 2004; Ekrt and Štech, 2008; Chang et al., 2013).
Interspecific gene flow may proceed only to the formation of F1 due to factors such as hybrid sterility. If hybrids reproduce, they may backcross with parents at the site of hybridization and create a hybrid swarm. Long-term hybrid swarms can be maintained in different ways (Anderson, 1949; Arnold, 1997). Spontaneous hybridization between the parental taxa might occur at a sufficiently high frequency to counterbalance selection against the hybrids and hybrid derivatives (Ellstrand et al., 2007). Alternatively, hybridization might be very rare, but the individuals of hybrid ancestry might be maintained largely by selective advantages (Ellstrand et al., 2007; Lee et al., 2012).
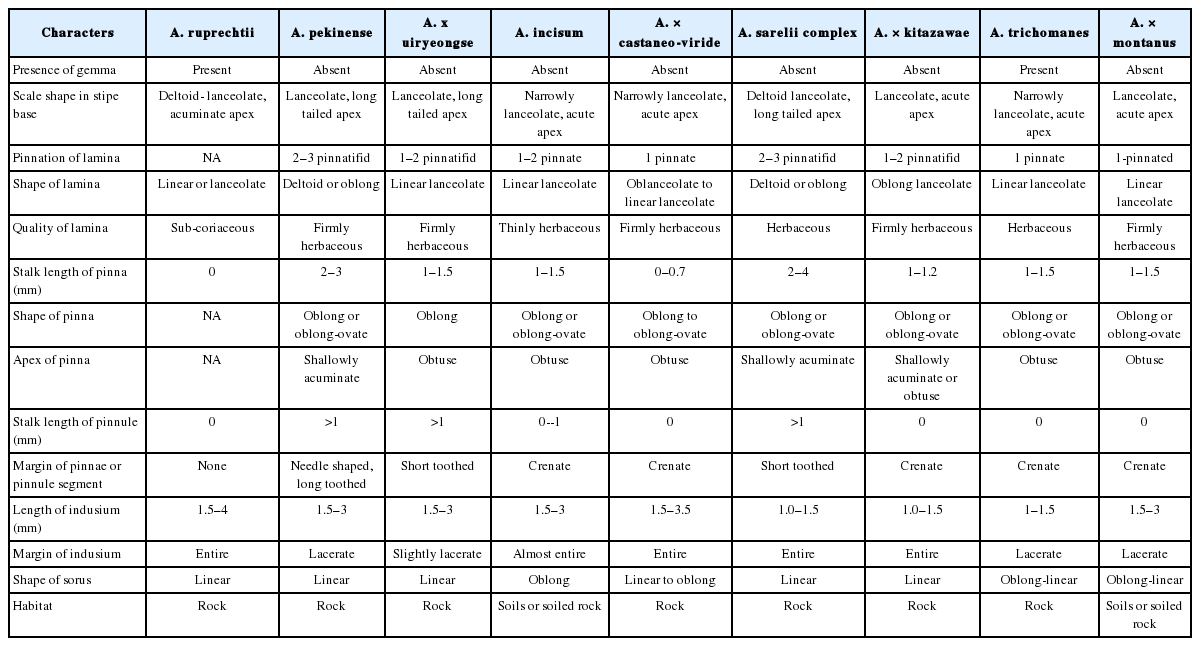
Asian walking fern, Asplenium ruprechtii Sa. Kurata, is characterized by simple leaf, almost smooth leaf margin, reticulate leaf vein; and produces a sterile hybrid or fertile allotetraploid hybrid with A. incisum Thunb. (Lovis et al., 1972; Ching and Iwatsuki, 1982). In Korean populations, it is hypothesized to form a hybrid, A. castaneo-viride Baker, between A. ruprechtii and A. incisum. Diverse ploidy levels of A. castaneo-viride has been reported: the populations of A. castaneo-viride in Mt. Mai and Mt. Duryun were diploid (2n = 72), whereas the population in Mt. Buram was tetraploid (2n=144) (Kwon et al., 2009). In addition to the above hybrid taxon, we have also found two new natural hybrids, A. × uiryeongse Lee & Lee between A. ruprechtii and A. pekinense Hance, and A. × montanus Lee & Lee which was postulated to be double hybrids between A. ruprechtii and A. trichomanes L., and one more with A. incisum (Lee et al., 2015). More recently, we have revealed unrecorded hybrid, A. × kitazawae Sa. Kurata & Hutoh, between A. ruprechtii and A. sarelii Hooker among 19 taxa in Korea (Lee et al., 2015).
Typical A. × uiryeongse has intermediate characteristics between the parents (A. ruprechtii and A. pekinense) such as a narrowly winged upper rachis, 1–2 pinnatifid lamina, shorter pinna stalk, shape of pinna and pinnule, and serration of indusium margins. A. castaneo-viride (A. ruprechtii and A. incisum) also exhibits parental morphology such as shape of basal pinna of lamina and preference of habitats (soils or rocks with soils). Intermediate characteristics of typical A. × kitazawae between the parents (A. ruprechtii and A. sarelii) and sympatric distribution with the parental species support hybrid origin. Shapes of scale in stipe bases, winged in upper rachis, 1–2 pinnatifid lamina, lamina quality, and shapes and margins of pinna strengthen the hybrid origin hypothesis (Lee and Lee, 2015, 2018; Lee et al., 2015).
Gross morphology of A. × montanus includes a winged upper rachis, one pinnate lamina, lamina quality, shape of pinna, and serration of indusium margins which are intermediate characteristics of the postulated parents (A. ruprechtii, A. trichomanes, and A. incisum). Documentation of hybridization has traditionally been based on morphological characters that are intermediate between the parental types or combine parental characters (Grant, 1981; Rieseberg et al., 1993). Hybrids can be identified easily by their aborted spores and intermediate morphology (Lovis and Reichstein, 1985; Jessen, 1995; Ekrt and Štech, 2008).
DNA content is often used as a proxy for ploidy levels in comparative studies of chromosome number variation (e.g., Ceccarelli et al., 1995; Suda et al., 2006; Suda and Travniček, 2006). Based on the assumption that DNA content varies with chromosome numbers when chromosome number increases due to whole- or partial-genome duplications (polyploidy or aneuploidy), this coupling of genome size with chromosome number should help to explain the ploidy levels of taxa and potential parental taxa of hybrids (Chung et al., 2012). Flow cytometry estimates reliable, comparative DNA content among samples using fresh leaves (Doležel and Bartoš, 2005).
DNA sequence data have been utilized to clarify parental taxa of hybrid origin groups. Chloroplast DNA has been instrumental in revealing interspecific hybridization and in documenting the hybrid origin region of several vascular plant species (Rieseberg, 1995; Arnold, 1997; Cronn et al., 2003; Lee et al., 2012). To infer maternal genetic histories rbcL, rps4-trnS, and rps4-trnS Intergenic spacers have provided critical information in many fern groups (Murakami et al., 1999; Skog et al., 2004; Wei et al., 2013).
In the present study, the postulated parental species of Asplenium hybrids are evaluated and hybrid processes are specified using cpDNA and cytological data. Putative parental species of Asplenium hybrid taxa have been hypothesized based on sympatric species and morphological characteristics: A. × uiryeongse (A. ruprechtii and A. pekinense), A. castaneo-viride (A. ruprechtii and A. incisum), A. × kitazawae (A. ruprechtii and A. sarelii), and A. × montanus (A. ruprechtii, A. trichomanes, and A. incisum). Flow cytometry evaluates a ploidy level for each taxon to clarify hybridization events, and cpDNA data reveal maternal lineages of each hybrid taxon. In addition, to reexamine the taxonomic delimitations and relationships of these taxa, we analyzed morphological, flow cytometry, and chloroplast DNA sequences data.
Materials and Methods
The sources of plant materials for morphology and DNA analysis including GenBank accession numbers are listed in Appendix 1. All voucher specimens were deposited at Ewha Womans University Herbarium (EWH). It was analyzed fourteen morphological characters observed and measured from mature individuals between each hybrid and the postulated parents of Asplenium (Lee et al., 2015).
Materials
Leaves used for DNA analyses were collected from the natural populations that encompass all morphological and habitat diversity. A total of five species of Asplenium and four putative hybrid, A. × castaneo-viride, A. × uiryeongse, A. × montanus, and A. × kitazawae were sampled. Three species of Asplenium, A. oblongifolium (from New Zealand AY283231/EU240026), A. lamprophyllum (from New Zealand AY283230/EU240021), and A. yoshinagae (from China, AY725030/AY725045) were used as outgroup, we got each three accessions of rbcL and rps4-trnS regions from DNA bank (Perrie and Brownsey, 2005; Li and Lu, 2006; Shepherd et al., 2008).
Cytological data (flow cytometry)
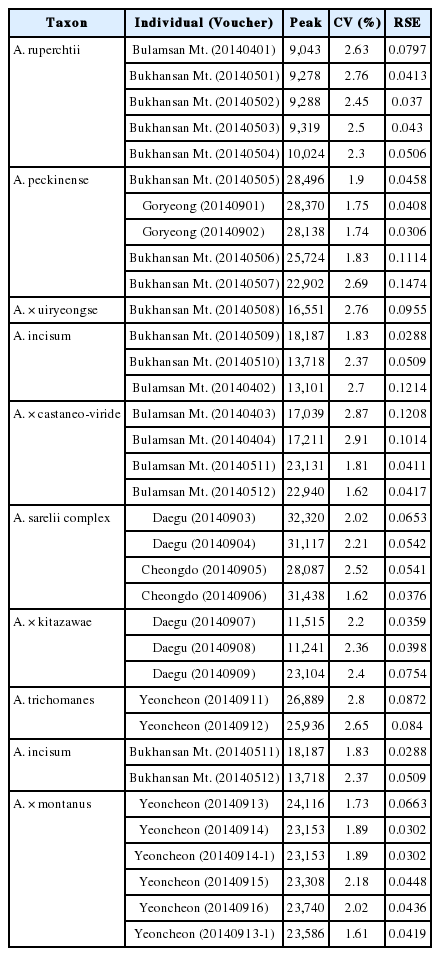
Young, fresh leaves of each samples, which they were same populations for DNA samples as Appendix 2, were stored in plastic bags at 4°C and their DNA ploidy level was determined. Diploid sample of Asplenium ruprechtii, verified by chromosome counting, was used as an internal standard. About 100 mg of fresh young leaf tissue was chopped using a fresh razor blade, in a Petri dish containing 500 μL ice-cold nucleic extraction buffer (solution B kit, Partec, Munster, Germany). The suspension was filtered through CellTris (Partec). After incubation period (10 min at room temperature), the staining solution containing 2 mL of DAPI (solution B kit, Partec) was added. After staining, data analyzing was recorded using the cytometer, CyFlow Ploidy Analyser (Partec GmbH).
Ploidy levels size were estimated as the mean of individual counts for A. × castaneo-viride, A. × uiryeongse, A. × kitazawae and A. × montanus, and related parents’ taxa comparing with means values of standard (Asplenium ruprechtii, 2x) (Yan et al., 2016). The coefficients of variation for each analyzed sample were calculated. Moreover, we selected less than 3 of coefficient of variation (CV), and less than 0.2 of relative standard error (RSE). Measurements were performed at least two to three times per individual on different days, and in each run 5,000 nuclei were recorded within both the standard and sample peaks. Mean and CV values of each peak were calculated using WinMDI 2.8 (Purdue University Cytometry Laboratories, West Lafayette, IN, USA). The Appendix 2 presents means and standard deviations of Mean peak, CV, and RSE values of the samples.
Molecular data (DNA extraction, sequencing, and analyses)
Total genomic DNA was extracted using the DNeasy Plant Mini Kit (Qiagen Inc., Valencia, CA, USA) following the manufacturer’s instructions. Polymerase chain reaction (PCR) amplification, purifications, and DNA sequencing strategies followed (White et al., 1990). The amplification reaction mixture was prepared using TaKaRa Ex Taq DNA polymerase (Takara Bio, Tokyo, Japan). For the cpDNA phylogeny, a total of 30 accessions, including three or two individuals of A. × castaneo-viride, A. × uiryeongse, A. × kitazawae, and A. × montanus for two cpDNA regions, rbcL, rps4-trnS, and rps4-trnS intergenic spacer, was analyzed.
Primers used for amplification and sequencing were trnS (TAC CGA GGG TTC GAA TC) (Souza-Chies et al., 1997; Smith and Cranfill, 2002) the rps4F1 (RPS4F1 GCC GCT AGA CAA TTA GTC AAT C) (Hennequin et al., 2003) for part of the rps4-trnS gene and rps4-trnS intergenic spacer (Schneider et al., 2004a), rbcL-TKT-F1 and rbcL-TKT-R3N-2 for the rbcL gene (Ebihara et al., 2005). Amplification was conducted using a PTC-100 thermal cycler (MJ Research, USA) with the following temperature profile for all regions: a 32 to 37 cycle reaction with denaturalization at 94°C for 1 min, annealing at 54°C for 1 min and extension at 72°C for 2 to 3 min. In addition, an initial denaturalization at 94°C for 2.5 min and a final extension at 72°C for 10 min were performed. PCR products were purified with AccuPrep PCR Purification Kit (Bioneer Inc, Daejeon, Korea). Automated sequencing analysis was performed using a sequencer (Base station, MJ Research, USA). Chloroplast DNA regions were each aligned with gap adjustments, using the Clustal X program, followed by manual adjustment (Gibson et al., 1994; Thompson et al., 1997). Each cpDNA region was analyzed using maximum parsimony (MP) by PAUP* (Swofford, 2002). Bootstrap values were calculated from 5,000 replicate analyses using tree bisection and reconnection branching swapping and simple stepwise addition of taxa (Felsenstein, 1985). PAUP* evaluated congruence the cpDNA sequence data using the incongruence length difference (ILD) test (Farris et al., 1994, 1995).
Results and Discussion
Morphological analysis
Asplenium × uiryeongse and its putative parental species, A. ruprechtii and A. pekinense, in natural habitats are shown in Fig. 1. Typical A. × uiryeongse has the intermediate characters of the parents such as 1–2 pinnatifid lamina, 1–1.5 mm stalk length of pinna, short toothed pinna, and slightly lacerate margins. Furthermore, A. × uiryeongse has some characteristics of A. ruprechtii, such as almost entire scale margins and linear lanceolate lamina. It also has characters similar to A. pekinense, such as lanceolate, long tailed apex of scales in stipe bases, firmly herbaceous lamina, and no gemma (Table 1) (Lee et al., 2015). Therefore, morphological data support Asplenium × uiryeongse as hybrid between A. ruprechtii and A. pekinense.

Sympatric natural habitats of four Asplenium hybrids and putative parental species. A. A. × uiryeongse (1) with A. ruprechtii (2) and A. pekinense (3). B. A. × castaneo-viride (4) with A. ruprechtii (2) and A. incisum (5). C. A. × kitazawae (6) with A. ruprechtii (2) and A. sarelii complex (7). D. A. × montanus (8) with A. ruprechtii (2), A. trichomanes (9), and A. incisum (5).
A. castaneo-viride (A. ruprechtii and A. incisum) has intermediate characters of the parental species such as herbaceous lamina, short toothed margin of pinnae, and almost entire margins of indusia (Fig. 1, Table 1). In the previous study, the morphological characters such as involving leaves, spores, epidermal cells, stomata and chromosome number support the hybrids between A. ruprechtii and A. incisum (Kwon et al., 2009). The accumulated morphological data clarify the hybrid origin of A. castaneo-viride from A. ruprechtii and A. incisum.
A. × kitazawae and its putative parental species, A. ruprechtii and A. sarelii, are presented in Fig. 1 and Table 1. Typical A. × kitazawae has intermediate characters of the parents such as acute apex of scale in stipe, 1–2 pinnatifid lamina, firmly herbaceous lamina, 1 mm pinna length, and crenate pinnae. The hybrid has both parental characters. Some characteristics such as no pinnule and lanceolate lamina share with of A. ruprechtii, and oblong or oblong-ovate pinna, shallowly acuminate pinna apex, 1–1.5 mm length of indusia, and missing gemma are similar to A. sarelii complex (Table 1) (Lee et al., 2015). Therefore, morphological data support A. × kitazawae as the hybrid between A. ruprechtii and A. sarelii.
A. × montanus and its putative three parental species, (A. ruprechtii, A. trichomanes, and A. incisum) in natural habitats are exhibited in Fig. 1. Typical A. × montanus has intermediate characters of the parents such as firmly herbaceous lamina, 1–3 mm length of indusia. A. × montanus has some characteristics of A. incisum, such as no gemma, habitat on soils or rock. It also has characters similar to A. trichomanes, such as 1-pinnated lamina, no pinnule, lacerate margin of indusia, and oblong-linear sori (Table 1) (Lee et al., 2015). Therefore, morphological data support A. × montanus as hybrid between A. ruprechtii and A. trichomanes, and once more hybridization between A. incisum.
Hybrids are known to have morphological characters intermediate between their parents and/or combining characters from each of the parents, and sometimes the hybrids display characters not found in either parent (Grant, 1981; Rieseberg et al., 1993). Our morphological results could conserve the hybridization from putative parental taxa.
Ploidy level analysis
Flow cytometry analysis detected diploid, triploid, tetraploid, and hexaploid plants (Figs. 2, 3, Table 2, Appendices 1, 2). Ploidy levels were estimated as the mean of individual counts for each taxon.

Flow cytometry histograms of two hybrids, Asplenium × uiryeongse (A. ruprechtii and A. pekinense), and A. × castaneo-viride (A. ruprechtii and A. incisum).

Flow cytometry histograms of two hybrids, Asplenium × kitazawae (A. ruprechtii and A. sarelii) and A. × montanus (A. ruprechtii, A. trichomanes, and A. incisum).
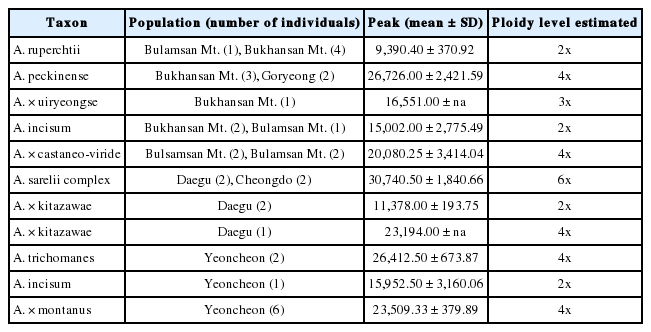
Nuclear DNA content and diploid level in examined populations of between the four hybrid taxa and related taxa within the genus Asplenium.
Five samples of Asplenium ruprechtii from two localities (Bulamsan Mt. and Bukhansan Mt.) were determined as diploids (Fig. 2, Table 2, Appendix 2). The species occurs throughout the Korea peninsula and is hypothesized as one of parental species of all the hybrids (Lee et al., 2015; Lee and Lee, 2018). The flow cytometry analyses clarified the ploidy level of the species as only a diploid.
The hybrid, A. × uiryeongse observed in the Bukhansan Mt. site, was a triploid (Fig. 2, Table 2, Appendix 2). Five samples of A. pekinense, the sympatric species, from two natural populations (Bukhansan Mt. and Goryeong) were tetraploids. This result fail to reject the hybrid hypothesis of A. x uiryeongse, derived from its putative and sympatric parental species, A. ruprechtii and A. pekinense (Lee et al., 2015).
Four samples of A. × castaneo-viride from two localities (Bulamsan Mt. and Bukhansan Mt.) were tetraploids in the flow cytometry analyses (Fig. 2, Table 2, Appendix 2). The results are incongruent from the previous reports. The hybrid was known as two ploidy levels: 2n = 72 (2x) from Maisan Mt. and Dureunsan Mt. populations (Kwon et al., 2009) and 2n = 144 (4x) from Bulamsan Mt. populations (Lee et al., 2015). Five samples of A. incisum from the same two localities (Bulamsan Mt. and Bukhansan Mt.) show diploids in the flow cytometry analyses (Fig. 2, Table 2, Appendix 1).
A. × kitazawae and its putative parental species, A. ruprechtii (2x) and A. sarelii complex (6x), were diploids or allotetraploids. Four samples of A. sarelii complex from two localities (Daegu and Cheongdo) were hexaploidy (Fig. 3, Table 2, Appendix 1). A. sarelii has been known as a diploid in China and tetraploids in Korea and Japan, but tetraploid A. anogrammoides only distributes in Korea (Lin and Viane, 2012). However, A. anogrammoides known as tetraploid in Korea were observed as a hexaploid in this study. It needs to conduct further research on Korean A. sarelii complex, including A. sarelii, A. anogrammoides, and A. pekinense covering all the distribution areas.
Six samples of A. × montanus from one locality (Yeoncheon) and its putative three parental species (A. trichomanes, A. ruprechtii, and A. incisum) were tetraploids (Fig. 3, Table 2, Appendix 2). Based on individuals with intermediate characters between A. ruprechtii and A. trichomanes and hybrid individuals with ecological characters of with A. incisum, double hybridization events have been hypothesized (Lee et al., 2015). These FCM results fail to deny the hypotheses that the hybrid, A. × montanus came out from its putative parental species, A. ruprechtii and A. trichomanes; and once more hybridized with A. incisum (Lee et al., 2015).
These flow cytometry results could sustain the hybridization among putative, sympatric parental taxa based on comparative genome size (ploidy levels).
Molecular data analysis
The aligned length of rbcL region is 1,205 bp, and the aligned length of rps4 gene and rps4-trnS intergenic spacer (IGS) region is 753bp. Of the 1,205 aligned rbcL region, 1,051 sites (87.2%) are identical, 43 sites (3.56%) are parsimony uninformative, and 111 sites (9.2%) provide phylogenetic information. MP analysis of the entire rbcL sequences finds 248 equally most parsimonious trees with a tree length (TL) of 191, a consistency index (CI) of 0.8534 (0.8100 excluding uninformative characters) and a retention index (RI) of 0.9622. One of 191 equally most parsimonious trees is shown in Fig. 4 and identical to the ones treating gaps with missing data.

Phylogenetic tree of four hybrids of Asplenium and related taxa deduced from rbcL data. Bootstrap values (>50%) by maximum parsimony method are shown above branches. Asplenium oblongifolium and A. lamprophyllum are used as outgroups.
In the 753 bp aligned rps4 gene and rps4-trnS IGS region, 550 sites (73%) are identical, 43 sites (5.7%) are parsimony uninformative, and 160 sites (21.2%) provide phylogenetic information. MP analysis of the entire rps4 gene and rps4-trnS IGS sequences finds 267 equally most parsimonious trees with a TL of 6, with a high CI (0.8764 or 0.8520 excluding uninformative characters) and RI (0.9513). One of 6 equally most parsimonious trees is Fig. 5.
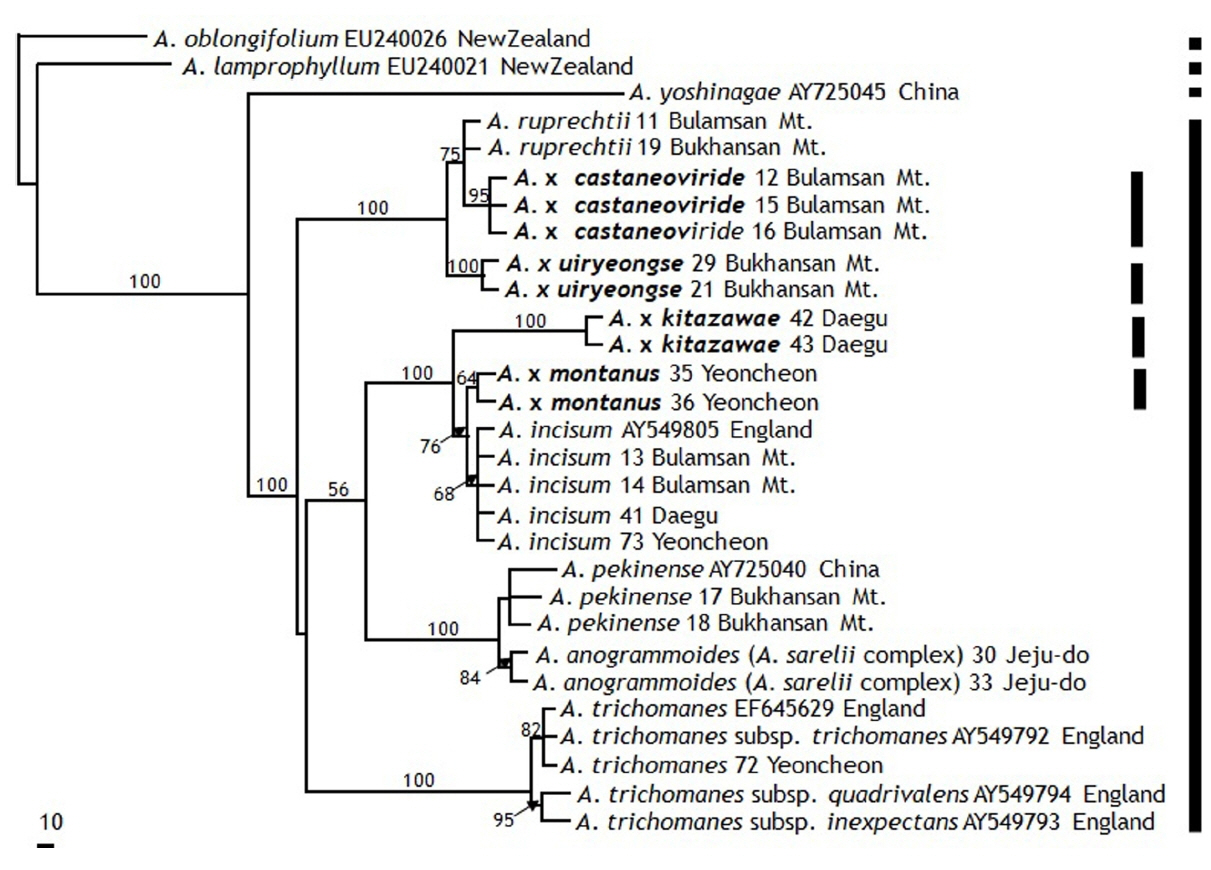
Phylogenetic tree of four hybrids of Asplenium and related taxa deduced from rps4-trnS and rps4-trnS intergenic spacer data. Bootstrap values (> 50%) by maximum parsimony method are shown above branches. Asplenium oblongifolium and A. lamprophyllum are used as outgroups.
The bounded DNA data of chloroplast genes rbcL and rps4 gene and rps4-trnS IGS for 25 accessions are ranged from 1,839 bp in A. trichomanes to 1,920 bp in A. incisum. The ILD p-value for the combined data set = 0.01, failing to prove that the data sets are homologous. The incongruence of the two cpDNA data sets suggests diverse, complex evolutionary history of maternal lineages. For the incongruence of the data sets, the combined cpDNA data is excluded in further phylogenetic analyses.
In the rbcL analyses, A. × ruprechtii and four hybrids (A. × uiryeongse, A. castaneo-viride, A. × kitazawae, and A. × montanus) form a clade with the strong support of a 100% bootstrap value (Fig. 4). However, A. ruprechtii exhibits three haplotypes refracting geographic patterns: Japan, Germany, and Korea types. Among the three haplotypes, Germany and Japan types form polytomy with A. × uiryeongse, A. × kitazawae, and A. × montanus; and Korean type is nested with A. × castaneo-viride. Especially, in MP trees, A. × castaneo-viride nested strongly with Korean A. ruprechtii with a 100% bootstrap value (Fig. 4). In addition, the same haplotype is found in A. ruprechtii (individuals 11, 19), which is identical to three individuals of A. × castaneo-viride (individuals 12, 15, and 16). From these results, we suggest that A. ruprechtii is a maternal parent of four hybrids.
A. incisum shows two haplotypes whereas only one haplotype is found in A. trichomanes. The sequencing results obtained from DNA bank are similar to the newly generated data from a Korean population, but one accession (AB574866) of A. pekinense from Japan is nested with A. anogrammoides (AB853879) from Japan. Two accessions of A. sarelii (AB574873 and AB014693) from Japan are nested with Korean A. anogrammoides (30 and 33). It suggests that A. sarelii and A. anogrammoides from Japan might be confused. Two species has been recognized as A. sarelii only. However, A. sarelii exhibits 2x (China), and A. anogrammoides is 4x (Japan) (Wang et al., 2003). In addition, it is revealed for the first time that A. anogrammoides in Korea is 6x. In the phylogenetic tree, A. sarelii and A. anogrammoides fail to prove the monophyly (Fig. 5). The taxa have been problematic in terms of morphological delimitation of the species. These results show to need the reexamination of these groups.
As in the rbcL data, A. ruprechtii forms a clade with A. x uiryeongse and A. castaneo-viride in the rps4 gene and rps4-trnS IGS region. The haplotype of two hybrids (A. castaneo-viride and A. × uiryeongse) make a monophyletic group with a 100% bootstrap value (Fig. 5). From these results, we confirm that A. ruprechtii is the maternal parent of A. × uiryeongse and A. castaneo-viride. However, not like in the rbcL topology, A. × montanus is distinctly nested with A. incisum as well as A. × kitazawae. It suggests that A. incisum should be another maternal parent of A. x montanus and A. × kitazawae. A. × kitazawae has been considered as the hybrid of A. ruprechtii and A. sarelii complex (A. sarelii and A. anogrammoides), but our results suggest another maternal linage of A. incisum. In addition, because of unclear taxon delimitation of A. sarelii complex, it is crucial to examine A. × kitazawae hybrid origins including A. sarelii complex. To clarify taxonomic statuses of A. × kitazawae and A. sarelii, further study with broader samplings covering distribution areas are preferred.
The cpDNA data suggest that maternal parents of four hybrids (A. castaneo-viride, A. × uiryeongse, A. × kitazawae and A. × montanus) are A. ruprechtii in common, and A. incisum should be another maternal parent of A. × kitazawae and A. × montanus. A. × kitazawae and A. × montanus might have been hybridized more than once. These results are also supported by morphological features (Lee et al., 2015), chromosome numbers, and spore characters (Kwon et al., 2009), except for A. × kitazawae. Further research with molecular markers and taxon sampling should be conducted to clarify both paternal and maternal lineages of the hybrids.
Acknowledgements
We thank Dr. Adjoa Ridhardson Ahedor at Rose State College for critical reading and comments on the earlier version of the manuscript. Flow Cytometry analyses were conducted at National Institute of Horticultural and Herbal Science with the technical assistance of H.-So. Kim (Breeding Technology Service Team).
Notes
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
Appendices
Appendix 1.
Collection data of four natural hybrid Asplenium taxa and related taxa studied in molecular analyses (*DNA accession No. used from GenBank). Voucher specimens were deposited at Ewha Womans University Herbarium (EWH). Numbers in bold after each accession number are the same as in Figs. 2–4. GenBank accession numbers listed in the following order: rbcL, *rps4-trnS, and rps4-trnS intergenic spacer.
Asplenium ruprechtii Sa. Kurata: [11], KOREA. Seoul: Bulamsan Mt., 16 Apr 2014, C. S. Lee 14041601 (EWH), MK774627, *MK774644; [19], Seoul: Bukhansan Mt., 19 May 2014, C. S. Lee 14051904 (EWH), MK774628, *MK774645.
A. × castaneo-viride Baker: [12, 15, 16], KOREA. Seoul: Bulamsan Mt., 16 Apr 2014, C. S. Lee 14041611-13 (EWH), MK774629, MK774630, MK774631, *MK774646, *MK774647, *MK774648.
A. × uiryeongse C. S. Lee & K. Lee; [21], KOREA. Seoul: Bukhansan Mt., 19 May 2014, C. S. Lee 14051906 (EWH), MK774632, *MK774649; [29], 12 Jun 2014, C. S. Lee & K. Lee 14061201 (EWH), MK774632, *MK774649.
A. × montanus C. S. Lee & K. Lee; [35, 36], KOREA. Gyeonggi-do: Yeoncheon-gun, 20 Sep 2014, C. S. Lee & K. Lee 14092005-6 (EWH), MK774615, MK774616, *MK774636, *MK774637.
A. × kitazawae Sa. Kurata & Hutoh in Kurata; [42, 43], KOREA. Gyeongsangbuk-do: Daegu, 25 Sep 2014, C. S. Lee & K. Lee 14092505-6 (EWH), MK774634, MK774635, *MK774651, *MK774652.
A. incisum Thunb.; [13, 14], KOREA. Seoul: Bulamsan Mt., 16 Apr 2014, C. S. Lee 14041605-06 (EWH), MK774620, MK774621, *MK995098, *MK995099; [41], Gyeongsangbuk-do: Daegu, 25 Sep 2014, C. S. Lee & K. Lee 14092508-09 (EWH), *MK995100; [73], Gyeonggi-do: Yeoncheon-gun, 20 Sep 2014, C. S. Lee & K. Lee 14092011 (EWH), MK774622, *MK774639.
A. pekinense Hance; [17, 18], KOREA. Seoul: Bukhansan Mt., 19 May 2014, C. S. Lee 140519010-11 (EWH), MK774623, MK774624, *MK774640, *MK774641.
A. trichomanes L.; [39, 40, 72], KOREA. Gyeonggi-do: Yeoncheon-gun, 20 Sep 2014, C. S. Lee & K. Lee 14092015-7 (EWH), MK774618, MK774619, MK774617, *MK774638.
A. anogrammoides (A. sarelii complex); [30, 33], KOREA. Gyeongsangbuk-do: Daegu, 25 Sep 2014, C. S. Lee & K. Lee 14092511-12 (EWH), MK774625, MK774626, *MK774642, *MK774643.