한국남부 자생 닭의난초 (난초과)의 시 공간에 따른 결실률 변이
Spatial and temporal variation on fruit set in Epipactis thunbergii (Orchidaceae) from southern Korea
Article information
Abstract
난초 열매의 결실의 시·공간적 변화가 장기적으로 개체군의 생존에 영향을 끼칠 수 있으며 수 세대에 걸쳐 유전적 다양성에 영향을 미칠 것이다. 본 연구의 목적은 화밀을 분비하는 육상 난초인 닭의난초를 대상으로 자연 조건에서 시·공간의 결실 수준을 조사하고 교배계를 파악하는 데 있다. 본 연구자들은 전라남도 해남군 징의리 해안 1.5 km 걸쳐 위치한 4곳 집단에서 2년간의 조사 기간 동안 수분실험을 행하였다. 2년 동안 결실률이 집단 내 작은 슈트의 모임인 패치 내에서 유사했다. 대조적으로, 패치 간에는 결실룰이 유의 한 수준에서 차이를 보였다. 또한 큰 화서를 지닌 식물이 작은 화서를 지니는 식물보다 훨씬 더 많은 열매 생성을 보였다. 닭의난초의 결실률은 2 년의 조사 기간 동안 비슷하였으나(14.1%) 연구된 보상을 주는(rewarding) 난초84종의 평균 결실률(37.1%) 보다 낮았다. 인위적인 자가 수분(90.5−95.2%), 인위적인 隣花수분(geitonogamy: 94.7−95.0%), 그리고 인위적인 타가 수분(91.3−91.4%)등에 의해서는 결실률이 상당한 수준으로 증가되었다. 꽃피기 전 수술을 제거 한 경우에 열매가 전혀 맺히지 않았으며 꽃 스스로 자동으로 수분이 일어나지 않았다. 본 연구 결과에 의하면 닭의난초는 자가화합성이고, 열매를 생성하기 위해서는 수분매개체인 곤충류가 필요하며, 2년간 조사 기간 중 징의리 집단들의 환경이 비슷하였음을 암시하고 있다. 본 연구 결과는 결실률이 시간보다 공간적으로 더욱 뚜렷하다는 점을 강조하고 있다.
Trans Abstract
Spatio-temporal variation in fruit set in orchids would affect long-term population viability and will influence genetic diversity over many generations. The aim of this study was to examine the breeding system of the nectariferous terrestrial orchid Epipactis thunbergii, to specifically determine levels of fruit set in terms of time and space under natural conditions. We examined pollination under natural conditions and conducted hand pollination experiments during a 2-year survey in four populations located along 1.5 km of coastal line in Jingui-ri (rual village) [Jeollanam-do (province), southern Korea]. We found that, over a 2-year period, levels of percentage of fruit set were similar within patches of the four populations. By contrast, we detected significant differences in the percentage of fruit set among patches. We also found that plants with larger inflorescence size produced significantly more fruits than plants with fewer flowers. Over a 2-year period, the percentage of fruit set for E. thunbergii was similar but low (14.1%) compared to that averaged for eighty-four rewarding species (37.1%). However, an increase in fruit set was achieved by hand-pollinations: artificial self-pollination (90.5−95.2%), artificial geitonogamy (94.7−95.0%), and cross-pollination (artificial xenogamy, 91.3−91.4%). No emasculated flowers produced fruits and no automatic pollination was found in E. thunbergii. Our findings suggest that E. thunbergii is a self-compatible terrestrial orchid that depends on pollinators (insects) to achieve fruit set in natural habitats, and that local environmental conditions were similar over a period of 2 years in the study area. Our results also highlight the cryptic variation of fruit production in time, but more pronounced variability in space.
Fruit set data are essential to the understanding of the evolution of floral characteristics in orchids (and other plants). A recent review for orchid reproductive biology (using data from complied from 216 non-autogamous orchid species in 92 genera) demonstrates that fruit production (i.e., female reproductive success) is generally caused by pollination limitation (Tremblay et al., 2005). A variety of biological and ecological variables such as pollinator availability, pollinarium removal and deposition, pollinator abundance and diversity, pollen quality and quantity, and breeding systems (self-compatibility or -incompatibility) may affect the fruit production, though these are generally not correlated with the degree of pollinator activity (Tremblay et al., 2005 and references therein). In addition, various ecological factors such as phenology, inflorescence size, inflorescence effects coupled with pollinator foraging behavior, conditions of microhabitats, density effects, population size effects, human disturbance, habitat fragmentation, environmental perturbations, weather conditions, and herbivory may affect reproductive success (Tremblay et al., 2005 and references therein). Studies on factors causing pollinator limitation (consequently, resulting in a generally high percentage of fruit-set failure) in many orchids suggest that pollination efficiency or pollen limitation would be the most important selection pressure on the evolution of their floral morphology (Dafni and Bernhardt, 1990; Neiland and Wilcock, 1998; Maad and Nilsson, 2004; Tremblay et al., 2005). Thus, many researchers often argue that “adaptive radiation” of pollinators for outcrossing taxa would be a major factor for the great taxonomic diversification of the Orchidaceae (Maad and Nilsson, 2004; Tremblay et al., 2005).
Understanding the variation of fruit set of orchids over space and time may be important, since heterogeneity in fruit set among populations and over time is just beginning to be recognized as potentially affecting evolutionary processes. As in many orchids fruit set is generally low, a year-to-year variation in fruit production in small populations of orchids due to possible fluctuations in environmental conditions would cause demographic stochasticity (Jacquemyn et al., 2009). These demographic variations in reproductive success within orchid populations, in particular in small ones (those of greatest conservation concern), could negatively influence effective population size (Ne, the number of effective breeding adults in a generation) over time. Theoretically, the degree to which random genetic drift changes allele frequencies, increases inbreeding, and decreases genetic diversity is an inverse function of Ne. The smaller Ne, the higher the probability that over generations loss or fixation of alleles in the population will be caused by genetic drift.
Owing to differences in ecological determinants in space, levels of fruit set usually vary among populations in a given species (Bino et al., 1982; Ackerman et al., 1997; Matsui et al., 2001; Ehlers et al., 2002; Chung and Chung, 2005; Jacquemyn and Brys, 2010). However, fruit set in some orchid populations is usually consistent from year to year (Nilsson, 1983; Gill, 1989; Primack and Hall, 1990; Ackerman and Moya, 1996; Tremblay et al., 2005), whereas the others revealed variation in fruit set over time due to environmental perturbations (Nilsson, 1983, 1984; Alexandersson and Ågren, 1996; Jacquemyn and Brys, 2010). Thus, more study is needed to better understand the spatio-temporal variation in fruit set in orchids, in particular rewarding orchids.
In this study, we selected the nectariferous terrestrial orchid Epipactis thunbergii A. Gray to investigate the differences in fruit set with reference to space and time, since populations of this species consist of variously sized patches, probably due to differences in clonal reproduction and seed establishment. If environmental conditions of microhabitats are fairly homogeneous in two or more consecutive years, we would expect a fairly consistency of fruit set during the periods. We may also predict that clumps with a large size would have higher fruit set than either solitary plants or clumps of relatively small number of shoots. This expectation is based on the assumptions that E. thunbergii has a rewarding pollination system and the pollinators’ tendency to visit more often larger floral displays (Tremblay et al., 2005 and references therein). Finally, we conducted artificial hand pollination experiments to determine whether a low fruit set in natural conditions is due to pollination limitation. Pollination limitation in plant populations may be detected experimentally when artificially enhanced pollination elevates fruit set above natural levels (Burd, 1994).
Materials and Methods
Plant material and study site
Epipactis thunbergii occurs in wet places of hill slopes in northeastern Asia (Japan, central to southern Korea, China, and Russian Far East; Kitamura et al., 1986). In southern Korea it is a rare species that usually grows in grasslands of coastal areas (M. Y. Chung and M. G. Chung, pers. obs). Epipactis thunbergii exhibits sympodial growth of a long, perennial rhizome; thus, the plants grow in clusters (Tatarenko and Kondo, 2003). The plant reaches a height of 30−70 cm, including inflorescence. Flowering in southern Korea commences in early to mid June and lasts for about one month. In general, a new flower opens daily and the inflorescences produce 2−27 flowers. The sepals (10−13 mm long) are green with red tint, and petals (9−12 mm long) are reddish-brown. The labellum (10−14 mm long) consists of two parts connected by a flexible constriction (Dressler, 1981). The outer part (epichile) is movable and tongue-shaped, and both sides are thickened forming a furrow with purplish-pink dots (Sugiura, 1996). The labellum’s proximal part (hypochile) is built like a concave gutter and its inside is white with purplish-pink veins on both lateral parts and orange-yellow raised markings on the middle part, from which nectar is secreted (Sugiura, 1996). The epichile plays a critical role in the pollination process: during retreating of a syrphid fly from the hypochile after sucking nectar, the forelegs of the fly press on both sides of the epichile furrow and simultaneously the epichile rises upward and the pollinarium attaches to the thorax of the fly (Sugiura, 1996). The two pollinia are granular and lack a caudicle. Each flower lasts for about 10 days, and 10 open flowers are found simultaneously on the same, large inflorescence. Fruits (about 2.0−2.5 cm long) contain large numbers of small seeds. Four species of syrphid flies (Diptera: Syrphidae; Sphaerophoria macrogaster, S. philanthus, Episyrphus balteatus, and Melanostoma scalare) were known as legitimate pollinators of E. thunbergii in Japan, with S. macrogaster regarded as the most effective pollinator (Sugiura, 1996).
On 17 June 2002, we studied four populations located along 1.5 km of coastal line in Jingui-ri (rural village), Hwangsan-myeon (township), Haenam-gun (county), Jeollanam-do (province) to examine breeding systems and spatial genetic structure of E. thunbergii at the landscape level. This area has been relatively well preserved compared to adjacent coastal areas in Hwangsan-myeon. In our study area, shoots of E. thunbergii are aggregated in patches, forming four main distinct spatial clusters, i.e. populations: (1) HEA (12×12 m, elevation ca. 10 m above sea level, asl, n = 113 all visually identified shoots) is located on a southeast-facing hillside where dwarf Pinus thunbergii and Eurya japonica are the dominant species; (2) HEB (12 × 20 m, elevation ca. 4 m asl, n = 63) is placed on a south-facing hillside where P. thunbergii, E. japonica, and Smilax china are the dominant species with a dense grass cover; (3) HEC (10 × 10 m, elevation ca. 7 m asl, n = 71) is also located on a south-facing hillside where P. thunbergii, E. japonica, and dwarf Fraxinus rhynchophylla are the dominant plant species; finally, (4) HED (8 × 11 m, elevation ca. 9 m asl, n = 58) is placed on a south-facing hillside where E. thunbergii grows on a relatively open grassland with a few P. thunbergii and E. japonica individuals.
Pollination in natural conditions
Since fruit-set is the most widely used measure of female reproductive success for orchids (Proctor and Harder, 1994; Neiland and Wilcock, 1998; Murren, 2002), we obtained information on the breeding system of Epipactis thunbergii from field observations of fruit-set and experimental pollination tests. Shoots of E. thunbergii grow in clusters probably due to long rhizomes. Hence, we designated each cluster as a patch and selected 11 spatially distinct patches in populations HEA and HED, respectively. To calculate the fruit set through open pollination, we mapped and marked all flowering shoots with red ribbons, and we counted the number of flowering shoots and the number of flowers per individual from the 22 patches during a 2-year study period (24−25 June and 12−13 July 2002, 26−28 June and 9−10 July 2003). To estimate the male reproductive success (Ashman, 1998), pollinia removal was checked from flowers during early (27 June, n = 58 and 47 in HEA and HED, respectively) and middle (10 July, n = 31 and 28 in HEA and HED, respectively) flowering periods in 2003. On 15 September 2002 and 17 September 2003, we counted the total number of fruits in the patches. In 2003, we failed to observe insect pollinators of E. thunbergii probably due to strong wind along the coast, even though careful observations were made during the day (08:00 to 18:00) for 20 h in total.
Hand pollination experiments
To investigate the breeding system of E. thunbergii, we conducted experimental pollination methods in populations HEB and HEC. Since E. thunbergii can also reproduce asexually through rhizomes every year, we assumed that each distinct patch had a different genotype (genet) in order to establish the xenogamous hand pollinations. Our ongoing study on clonal and fine-scale genetic structure in an allozymically relatively highly variable population, located at Oeogogae wetland in Mt. Jiri, revealed that the most spatially separated patches were genetically distinct (M. Y. Chung and M. G. Chung, unpubl. data). Inflorescences with several unopened flowers from 36 and 49 flowering shoots in HEB and HEC, respectively, were bagged with a fine mesh plastic net to exclude pollinators, from 27 to 30 June 2003. Fifteen (n = 165 flowers) and 14 shoots (n = 134) were used in HEB and HEC, respectively, for open pollination and, thus, they were not included for experimental pollinations. A total of 116 and 110 flowers for HEA and HEB, respectively, were randomly assigned to one of the five pollination treatments described by Dafni (1992): (i) emasculation without pollination to test the presence of agamospermy and to evaluate the rate of nonsexual reproduction; (ii) a test for spontaneous self-pollination to measure autogamy and the need for pollinators; (iii) artificial self-pollination to test self-compatibility by placing pollinia on the stigmas of the same flowers; (iv) artificial geitonogamy to evaluate self-compatibility between different flowers by placing pollinia on stigmas of adjacent flowers of the same inflorescence or different inflorescences belonging to the same genet; and (v) artificial xenogamy or cross-pollination to assess cross-compatibility. These experiments were separately conducted in the two populations from 08:00 to 18:00 during the 4-day period. On 18 July 2003, the bags were removed to minimize any artificial effects and fruit-set was assessed by counting the number of swollen ovaries.
Statistical analysis
We conducted Pearson correlation analysis to determine the correlation between the number of flowers and the number of fruits for all the data set (240 shoots) within populations. To determine local heterogeneity in fruit set within and among patches in HEA and HED populations surveyed in 2002 and 2003, we used G statistic for the log-likelihood ratio goodness of fit test (e.g., Matsui et al., 2001). Type I error was adjusted for multiple tests by Šidák (1967) method conducted within and among patches (á = 0.0015 and á = 0.0127, respectively).
The calculation of fruit set must be on a per plant basis - not per flower - otherwise the data are not independent and the analyses may suffer from pseudoreplication (Ackerman et al., 1997). On a per-patch basis, thus, we calculated means with standard deviation (SD) in the number of flowers, the number of fruits, and the percentage (%) of fruit set. We further used a two-way ANOVA to assess the effect of year, site and their interaction on the percentage of fruit (e.g., Jacquemyn and Brys, 2010).
Finally, statistical tests for differences in fruit set between artificial treatments were performed by obtaining 95% confidence intervals (95% CI: see Eq. 1) because the data are binomially distributed (Chung and Chung, 2003; C. Wilcock of University of Aberdeen, United Kingdom, pers. comm.).
where P = percentage of fruit set, and n = total number of flowers examined.
Results
Pollination in natural conditions
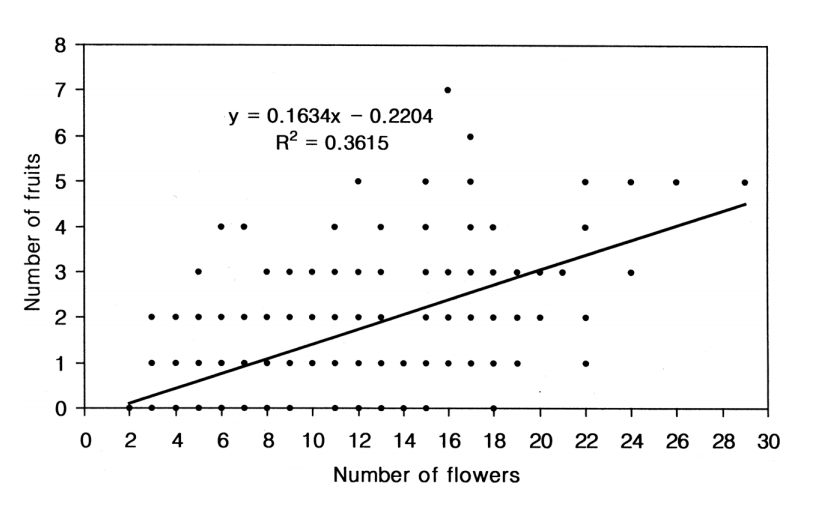
The correlation analysis, based on all the open-pollinated data set (n = 240), revealed that, overall, shoots with greater number of flowers produced significantly more fruits than shoots with fewer flowers (r = 0.601, R2= 0.362, P = 0.000; Fig. 1).
In open-pollinated conditions, fruit set within patches was generally homogeneous accordingly to G statistic for the loglikelihood ratio goodness of fit test. We found only two significant differences within the patches HEA-2 and HED-4 in 2002 (Table 1). In contrast, there were significant differences among 11 patches in two populations for the 2-year period (Table 1), which is in part due to the total absence of fruit set in HEA-1, HEA-9, HEA-11, HED-9, and HED-10 in 2002 and 2003 (Table 2). We found no significant correlation in the percentage of fruit-set between 2002 and 2003 in HEA, whereas we detected a significant correlation in HED (Spearman rank correlation analysis: rs= 0.85, P = 0.004; á = 0.025). Averaged within patches, the percentage of fruit set did not differ significantly between years (10 ± 7.20 and 10.9 ± 8.06 for 2002 and 2003, respectively in HEA; 17.1 ± 14.40 and 17.7 ± 10.87 for 2002 and 2003, respectively in HED; Table 2), but the fruit production in HED was significantly (P < 0.05) greater than that in HEA (Tables 2 and 3).

G statistic for the log-likelihood ratio goodness of fit test for local heterogeneity in fruit set within and among patches in HEA and HED populations of Epipactis thunbergii surveyed in 2002 and 2003. Type I error was adjusted for multiple tests by Šidák (1967) method conducted within and among patches (ά = 0.0015 and ά = 0.0127, respectively), * P < 0.05; na, no fruit production within patches.
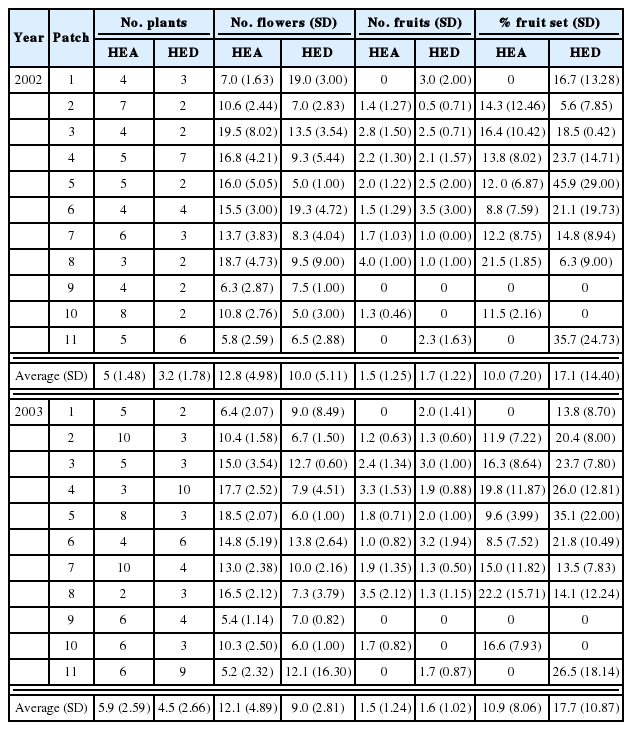
Fruit set per plant by open pollination among patches in HEA and HED populations of Epipactis thunbergii in 2002 and 2003. Values in number of flowers, number of fruits, and percentage of fruit set are means with SD (standard deviation in parentheses).
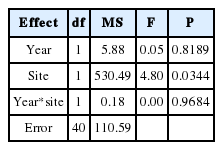
Analysis of variance (ANOVA) of the effects of year, site and their interaction on the percentage of fruit set of Epipactis thunbergii.
The entire removal of pollinia rarely occurred probably due to the granular characteristic of E. thunbergii pollinia. The percentages of the number of removed pollinia examined in HEA and HED were very low and similar to each other (4.31% [4 out of 89] and 8.00% [6 out of 75] in HEA and HED, respectively). These percentages were significantly lower than the levels of fruit set in natural conditions (Table 2).
The frequency of flower visits by any insect was extremely low in the four populations, probably due to strong wind in the coastal areas. No pollinators carrying pollinia were observed during the survey period. Limited observations included sweat bees (Lasioglossum sp.) collecting pollen using their forelegs and then loading pollen grains on the scopa of their hind legs. Nevertheless, they did not aid pollination. Several honey bees (Apis mellifera) landed and stayed for a while on the sepal and labellum but they never moved into the inner part of the flower or touched the gynostemium. Bombus diversus diversus touched flowers and went immediately away.
Hand pollination experiments
Although examined populations of E. thunbergii in this study showed very low levels of fruit set in natural conditions, a significant increase in fruit set was achieved through hand-pollination compared to open pollination (Table 4). Artificial self-pollination (90.5% and 95.2%), artificial geitonogamy (95.0% and 94.7%), and cross-pollination (artificial xenogamy, 91.3% and 91.4%) produced fruits for HEB and HEC, respectively (Table 4). There were no significant differences (95% CI did overlap; data not shown) in fruit set between induced autogamy and both artificial geitonogamy and artificial xenogamy, and between artificial geitonogamy and artificial xenogamy, indicating that E. thunbergii is highly self-compatible. In addition, hand pollination was not significantly different between the two populations, as 95% CI did overlap (data not shown). No emasculated flowers produced fruits and no automatic pollination was found in E. thunbergii. Since agamospermy and spontaneous autogamy were not detected in this study, pollinia vectors are essential for fruit set in natural habitats.
Discussion
Pollination in natural conditions
As predicted, we found a relatively homogeneous fruit production over a 2-year period. Tremblay et al. (2005) also noted that fruit production in orchids would be rather consistent from year to year. However, Nilsson (1978) found a significant temporal variation (5.0−19.8%) in fruit production of Epipactis palustris over a 3-year period. More recently, Jacquemyn and Brys (2010) also found significant variations (5.9−19.9%) in fruit set in the food-deceptive orchid Orchis purpurea in a grassland population over a 5-year period. As Jacquemyn and Brys (2010) stressed, we would find temporal variation of fruit set, if we study it over several consecutive years.
A similar level of fruit set was also found within patches of E. thunbergii, suggesting a leptokurtic distribution of pollinia dispersal of this species by journal insect pollinators. This leptokurtic distribution of between-plant insect flights would result in similar percentage of fruit set within patches of E. thunbergii, though we did not quantify pollen dispersal patterns. By contrast, fruit set varied among 11 patches in two populations for the 2-year period. We found no fruit set in the five patches (HEA-1, HEA-9, HEA-11, HED-9, and HED-10) entirely covered by the shrub Eurya japonica. Fruit set was significantly higher in HED than in HEA. This fruit-set heterogeneity in space is probably due to the fact that most shoots in HED grow in relatively open grassland habitats.
In orchids, the costs of flower longevity may be high due to nectar production (Ashman and Schoen, 1997). Neiland and Wilcock (1995) suggested that flower persistence in some European orchids has probably evolved in response to low pollinator visitation. Each flower of E. thunbergii lasts for about 10 days, and low pollinator visitation may be one reason why this species produces long-lived flowers. Supporting our second prediction, we found that shoots with a greater number of flowers (larger inflorescences) had significantly more fruits than shoots with fewer flowers. Prolonged flowers could be advantageous because it results in a large inflorescence display size (Murren and Ellison, 1996). Our results are consistent with several other orchid species where a large display attracts more pollinators and would increase reproductive success (Waite et al., 1991; Murren and Ellison, 1996; Ehlers et al., 2002; Murren, 2002; Stpiczyñska, 2003).
For a total of 34.9 hrs, Sugiura (1996) observed nine different pollinators: four legitimate pollinators plus five visitors such as one Diptera (Mesembrius flaviceps), two Hymenoptera (Leptothorax sp. and Lasioglossum scitulum), and one species of Thysanoptera. Among four legitimate pollinators of syrphid flies in Japan, Sugiura (1996) identified Sphraerophoria macrogaster as the most effective pollinator of Epipactis thunbergii. Epipactis palustris has as many as 103 species of potentially effective pollinators (Nilsson, 1978; Tremblay, 1992). However, we did not encounter any legitimate pollinator for a total of 44 hrs in the studied Korean populations of Epipactis thunbergii. We suspect that the failure to identify pollinators might be attributed to windy weather conditions in the coastal areas and/or probably paucity of insect fauna in the study areas. These factors may be associated with the three-fold lower levels of fruit set in the study areas than in Japan (10−17.7 % vs. 54.7%, respectively). Other species of Epipactis are pollinated by various insects such as wasps, bees, and flies: E. helleborine by Vespa spp. and Vespula spp. (Judd, 1972; Proctor and Yeo, 1973; Ehlers et al., 2002); E. palustris by Eumenes spp., Apis mellifera, and 12 other species of insects (Nilsson, 1978); E. consimilis by Sphaerophoria scripta and S. rueppellii (Ivry and Dafni, 1977), and E. pupurata by Baccha elongata, Vespula vulgaris, and V. rufa (figures are available at http://www.univ-lille1.fr/orchid by P. Watkin of University of Lille 1, France). Again, the documentation of a variety of pollinators in other Epipactis spp. may be related to higher fruit set in these species (mean percentage of fruit sets from congeners = 39.7%) compared to E. thunbergii in southern Korea. Furthermore, the percentage of fruit set found in E. thunbergii was considerably below the average for nectariferous orchids (average 50.8%, N = 30; Neiland and Wilcock, 1998) or rewarding orchids (average 37.1%, N = 84; Tremblay et al., 2005). A further survey, including nocturnal observations, is necessary to identify the pollinators of E. thunbergii in southern Korea.
Hand pollination experiments
Our artificial pollination experiments revealed a significant increase in fruit set compared to open pollination. As presented in Table 4, similar results were observed in Epipactis consimilis (Ivry and Dafni, 1977). Since a significant increase in fruit set was achieved through hand-pollination, and agamospermy and spontaneous autogamy were not detected in this study [as also found in E. consimilis from Israel; Table 4 (Ivry and Dafni, 1977) and E. helleborine (Ehlers et al., 2002)], pollinia vectors appear to be essential for fruit set in natural habitats of E. thunbergii. Pollinator limitation was evidenced, with only 6% of 164 flowers examined in this study having removed pollinia. This percentage of pollinia removal was considerably lower than those observed in E. palustris (60−67%, Nilsson, 1978) and E. helleborine (up to 85%, Ehlers et al., 2002) in Sweden. Unlike the present work, previous studies in other orchids revealed that the rate of pollinia removal is higher than the fruit set (Schemske, 1980; Proctor and Harder, 1995). The low rate of pollinia removal observed in the present study would be attributable to only a 2-day survey and/or rarity of pollinators during the study period.
Conservation implications
The breeding system of E. thunbergii suggests that survival and long-term persistence of the species would be seriously threatened if the interaction between this species and its pollinators continues to be disrupted. No fruit set was found in the five patches examined in the present study during the 2-year survey. Since these patches were entirely covered by dwarf Eurya japonica and vines of Smilax china, we suspect that the dense cover may prevent insect pollinators from visiting flowers of E. thunbergii. Thus, we recommend that branches near the ground should be regularly removed to make insect pollinators accessible to these patches allowing an increase in levels of fruit set. The lack of pollinators in the study areas is somewhat surprising, although we can speculate that a steady urbanization and increased use of pesticides in paddy fields nearby the study area would decrease the number of insects from year to year. Thus, we recommend conducting long-term monitoring of insect fauna in the areas of southern Korea where several orchid species grow.
Acknowledgements
The authors thank Y. D. Kang and D. Y. Cho for providing support and assistance in conducting experimental pollination during the study period. Special thank goes to Drs. Chris Wilcock, Mary Ruth Neiland, and Jordi López-Pujol for reading earlier versions of the manuscript and making helpful suggestions. Dr. Sugiura provided information of Epipactis thunbergii in Japan. This research was supported by a Korea Research Foundation grant (KRF-2013R1A1A2063524) to M.G. C.