Dryopteris melanocarpa (Dryopteridaceae), a new record of a fern species among the Korean flora of Jejudo Island
Article information
Abstract
Dryopteris melanocarpa Hayata is an unrecorded perennial monilophyte species in Korea that belongs to the genus Dryopteris of Dryopteridaceae. It was initially found to grow wild in a cave on Daerok Mountain, Jejudo Island and has been known to grow spontaneously only in Taiwan and Japan thus far. This species is morphologically very similar to D. sparsa (D. Don) Kuntze and D. subexaltata (Christ) C. Chr. However, D. melanocarpa is distinguished from those related taxa in that the stipe scale shape is broadly lanceolate and its lamina is triangularly lanceolate and herbaceous. In this study, in order to identify the D. melanocarpa, which was found on Jejudo Island, morphological and molecular analyses were conducted.
INTRODUCTION
Thirty-seven families and more than 12,000 species of monilophytes are known to be distributed worldwide (Kumar et al., 2022; Nitta et al., 2022). The family Dryopteridaceae, in particular, reportedly has 55 genera and more than 1,870 species (The Plant List, 2013). Within the genus Dryopteris Adans., approximately 400 species are predominantly distributed in East Asia (Zuo et al., 2022). Ferns in Korea show a distribution of 33 families and 75 genera, including monilophytes and lycophytes. Among ferns is the Dryopteridaceae family, which has been reported to include 82 taxa (Korea National Arboretum, 2022). The genus Dryopteris is distinguished from other genera of the Dryopteridaceae family based on the following characteristics: rhizomes short, ascending or erect; pinnatifid lamina with one pinnate leaf or if greater than one, the lower part of the pinna grows to be asymmetric; free leaf veins; and orbicular reniform indusia (Ohwi, 1965; eFloras, 2008; Ebihara, 2017). The taxa of the genus Dryopteris are known to be frequently polyploidized due to interspecific hybridization and apogamous (Hori 2017). As a result, substantial morphological variation occurs in identical taxa, and the traits that define a single taxon from related taxa are ambiguous. Therefore, scholars disagree on the taxonomic boundary (Lee and Park, 2013). Thus, reports have shown that 29 to 47 taxa are distributed in Korea (Lee, 2012; Kim et al., 2015; Lee and Lee, 2018; Korea National Arboretum, 2022).
In the phylogenetic studies of monilophytes, seven regions of chloroplast, atpA, atpB, matK, rbcL, rps4, trnL-F, and rps4-trnS, are commonly used (Nitta et al., 2022). The chloroplast gene, ribulose-bisphosphate carboxylase (rbcL), represents mostly widely and frequently sampled in phylogenetics of plants, and it is known to be suitable for inferring phylogenetic relationships of ferns (Hasebe et al., 1994; Nitta et al., 2020). It has also been shown that the combined use of coding regions such as rbcL and non-coding regions such as trnL-F leads to a higher resolving power compared to the use of a single region (de Groot et al., 2011; Nitta et al., 2020).
The reported species of sect. Nephrocystis that grow naturally in Korea are D. subexaltata (H. Christ) C. Chr., D. sparsa (Buch.-Ham. ex D. Don) Kuntze, D. sabaei (Franch. & Sav.) C. Chr., and D. maximowiczii (Baker) Kuntze (Park, 1975; Moon 2002; Lee and Lee, 2018; Son et al., 2019). These species share scales with flat bases on the stipe but not on the lamina as a feature (Ohwi, 1965; Moon et al., 2002). Dryopteris subexaltata has been presented as an unrecorded species based on its characteristic matured indusium that is irregularly lacerate, and its sori, which are attached to almost all the pinnae of the lamina (Moon et al., 2002). Dryopteris sparsa was initially detected in Mulchatoreum, Jeju-si, Jeju-do, and it shares with D. subexaltata the characteristic absence of scales on the costa. However, its indusium displays orbicular-reniform, and entire when matured, without lacerate, and it has been presented as an unrecorded species (Son et al., 2019). Dryopteris sabaei was recorded as having sori attached only to the pinna on the upper part of the lamina (Park, 1975), although this has not been confirmed in the distribution in Korea (Lee and Lee, 2018). The publications “Index to Ferns and Lycophytes in Japan” (Ebihara, 2017) and “Pteridophytes of Korea: Lycophytes and Ferns” (Lee and Lee, 2018) record that D. maximowiczii has three to four pinnate with the lamina in a broad and pentagram ovoid form. As this species differs from the aforementioned unrecorded species in terms of the morphological characteristics of the lamina, it is excluded from the present investigation.
In this study, we report D. melanocarpa Hayat as an unrecorded species in Korea based on our discoveries on Mt. Daerok, Jejudo Island. We here provide a description, photographs, and a key as basic data to distinguish this species from its related taxa.
MATERIALS AND METHODS
Sample collection
Dryopteris melanocarpa was discovered during a survey conducted on Jejudo Island from 2020 to 2022 to investigate monilophytes taxa within the family Dryopteridaceae. To distinguish it from related taxa in terms of morphology and molecular biology, we collected specimens and samples of D. sparsa from Mulchatoreum in Jeju-si and Gungdaeak in Seogwipo-si, and of D. subexaltata from Suak valley in Seogwipo-si (Appendix 1). The distribution of D. sabaei could not be confirmed in Korea. Voucher specimens of D. melanocarpa and its related taxa were prepared and stored in the specimen repository of the Warm-temperate and Subtropical Forest Research Center (WFRC).
Morphological study
The morphological characteristics of D. melanocarpa and its related taxa were observed using photographs taken with a Nikon camera (Nikon D750, Nikon Co., Japan), with particular attention given to the lamina, stipe scales, and sori. For the morphological features of D. sabaei, readers can refer to Ebihara (2017) and Ohwi (1965). The morphological descriptors followed the previous research on pteridophytes of Korea (Lee and Lee, 2018), and the morphological identification of each species was based on previous studies (Hayata, 1914; Moon et al., 2002; eFloras, 2008; Ebihara, 2017; Lee and Lee, 2018; Son et al., 2019).
Molecular phylogenetic study
The collected D. melanocarpa and its related taxa were used. Fresh tissue weighing 0.1 mg was finely ground and washed using STE buffer (5M sucrose 5 mL, 1 M Tris-HCl 3 mL, and 0.5 M EDTA 10 mL) to remove polysaccharides, polyphenols, and other impurities (Dempster et al., 1999). Subsequently, total DNA was extracted using the cetyltrimethylammonium bromide (CTAB) method (Dempster et al., 1999; Shepherd and McLay, 2011; Healey et al., 2014).
To amplify the trnL-F and rbcL regions of chloroplast DNA, the polymerase chain reaction (PCR) was employed using the primers “e” and “f” for trnL-F (Taberlet et al., 1991) and “aF” and “F1379R” for rbcL (Hasebe et al., 1994; Wolf et al., 1999). A PCR amplification cocktail was prepared with 2 μL of template DNA, 1 μL of each primer, 5 μL of 10× PCR buffer, 2 μL of dNTPs, and 0.5 μL of Taq polymerase (iTaq DNA Polymerase, iNtRON Biotechnology Inc., Seongnam, Korea) diluted with sterile distilled water to a total volume of 50 μL. A PCR cycler (Biometra T One 96G, Biometra GmbH, Göttingen, Germany) was used for amplification under the following PCR cycling conditions: pre-denaturation at 95°C for 3 min; 35 cycles of denaturation at 95°C for 1 min, annealing at 54°C for 40 s, and extension at 72°C for 45 s for trnL-F or for 90 s for rbcL; and final extension at 72°C for 7 min (Taberlet et al., 1991; Hasebe et al., 1994; Wolf et al., 1999; Lee and Park, 2013). The PCR products were visualized on a 1% agarose gel, and sequencing was outsourced to Macrogen Inc. (Seoul, Korea).
The obtained sequences were compared with the registered monilophyte sequences using the Basic Local Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast.cgi) from the National Center for Biotechnology Information (NCBI) website for taxonomic identification. The determined sequences were aligned using Clustal W (Thompson et al., 1994) of the software Molecular Evolutionary Genetics Analysis Version 11 (MEGA 11) (Tamura et al., 2021). The sequences analyzed of the materials used in this study, D. subexaltata (trnL-F: OR513049, rbcL: OR513052), D. sparsa (trnL-F: OR513048, rbcL: OR513051) and D. melanocarpa (trnL-F: OR513047, rbcL: OR513050) are registered in GenBank (https://submit.ncbi.nlm.nih.gov/) (Appendix 1). To investigate the molecular genetic relationships among D. melanocarpa and its related taxa, a phylogenetic analysis was conducted on a total of nine species including D. monticola (Makino) C. Chr. as an outgroup. The corresponding chloroplast DNA sequences of the rbcL and trnL-F regions were combined to construct a phylogenetic tree (Appendix 1). The analysis was performed using MEGA 11 software (Tamura et al., 2021) with the neighbor-joining method with 1,000 iterations to generate bootstrap supports. The reliability of the phylogenetic analysis was ensured using information on monilophytes obtained from NCBI.
RESULTS
Morphological study
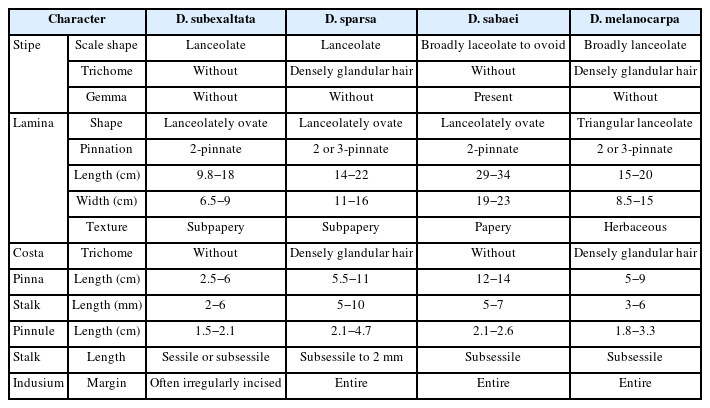
Dryopteris sparsa, D. subexaltata, and D. sabaei, which are closely related to D. melanocarpa exhibit the following morphological features: lanceolate basal stipe scales, 2-pinnate lamina, lanceolately ovate lamina, an absence of glandular hairs on the stipe and costa, and short or adnate pinnules and irregularly divided indusia. Dryopteris sparsa has lanceolate basal scales, 2- or 3-pinnate lamina, lanceolately ovate lamina, subpapery, and stalks on the pinnae and pinnules. It shares similarities with D. melanocarpa, such as densely glandular-haired stipe and costa and undivided indusia when the sori are matured. Dryopteris sabaei has broadly lanceolate to ovoid basal scales, 2-pinnate lamina, lanceolately ovate lamina, stalks on the pinnae and subsessile the pinnules, and gemma present at basal of the stipe (Ohwi, 1965; Ebihara, 2017). However, D. melanocarpa has broadly lanceolate stipe scales, 2- or 3-pinnate lamina, triangular lanceolate lamina, herbaceous, and stalks on the pinnae and pinnules. These distinctive characteristics clearly differentiate D. melanocarpa from the related taxa (Fig. 1, Table 1).

Photographs of Dryopteris melanocarpa Hayata. A. Habit. B. Scale at basal parts. C. Upper parts of stripe. D. Glandular hairs at costa of adaxial. E. Glandular hairs at costa of abaxial. F. Stalk of basiscopic pinnules. G. Immature sori and indusium. H. Shape of pinnule. I. Sori. J. Lamina of D. sparsa on Mulchatoreum in Jeju-si. K. Lamina of D. sparsa on Gungdaeak in Seogwipo-si.
Molecular phylogenetic study
Dryopteris melanocarpa and related taxa collected from Jejudo Island were determined to be identical to registered foreign species. This was based on the 99.89–100% sequence homology of the trnL-F and rbcL regions in the NCBI data. It should also be noted that D. subexaltata showed 100% homology with the registered foreign D. subexaltata and D. hayatae Tagawa, indicating a close genetic relationship between the two species. To explore the phylogenetic relationships of D. melanocarpa, the species under study, with its related taxa, D. sparsa, D. subexaltata, and D. sabaei based on the differences in chloroplast DNA sequences, we analyzed the phylogenetic relationships among the three species sampled on Jejudo Island and five species whose sequences were obtained from the NCBI database. This analysis resulted in their classification into five distinct groups. D. subexaltata-Jeju formed the one group with and D. subexaltata-Korea (Fig. 2, Group 1) and D. melanocarpa-Jeju formed the second group with D. melanocarpa-Japan (Fig. 2, Group 2). D. sparsa-Jeju was closely related to D. sparsa-Japan and formed the third group (Fig. 2, Group. 3). Dryopteris sparsa-China showed a distant relationship to species distributed on Jejudo Island and in Japan and formed the fourth group (Fig. 2, Group 4). Dryopteris sabaei formed the fifth group (Fig. 2, Group 5). The results of this study clearly demonstrate significant variations in both the morphological and molecular genetic dimensions of the phylogenetic analysis (Fig. 2).

Phylogenetic tree based on the sequence of Dryopteris subexaltata, D. sparsa, D. sabaei, and D. melanocarpa using the primer set trnL-F and rbcL (see Appendix 1). Bootstrap support values are indicated on the nodes.
Taxonomic Treatment
Dryopteris melanocarpa Hayata, Icon. Pl. Formosan. 4: 163, f. 104, 1914 (Fig. 1).—TYPE: Taiwan. Chiayi county, Mt. Arisan, Jan 1912, B. Hayata & S. Sasaki s.n. (syntypes: TAIF!, 1042, 1043).
Korean name: Geom-eun-jok-je-bi-go-sa-ri (검은족제비고사리).
Evergreen herb, 25−40 cm tall. Rhizomes short, ascending, or erect. Fronds monomorphic; stipe 13−19 cm long, with densely glandular hairs, sparsely scaly; scales membranous, light brown, broadly lanceolate. Lamina 2- or 3-pinnate, herbaceous, triangular lanceolate, yellowish green to bright green, 15−20 cm long. Lowest pinna asymmetric triangle, triangular lanceolate, 5−9 cm long, stalk 3−6 mm long, glandular hair densely on the costa. Basal pinnule largest, triangular ovate, 1.8−3.3 cm long, stalk subsessile, margin serrate, slightly undulate. Sori dorsal, on middle of veinlets; indusium grayish white, orbicular-reniform, entire; spores matured brown to black.
Distribution: Korea (Jejudo Island), Taiwan (almost all regions), Japan (Pref. Fukuoka, Kagoshima, Wakayama, and Mie).
Habitat: Moist area of a cave entrance zone.
Specimens examined: KOREA. Jeju-do: Seogwipo-si, Pyoseom-myeon, Gasi-ri, Mt. Daeroksan, 2 Jun 2021, Soon Yeol Ko 10034257 (WFRC; 2 sheets).
Ecology: Dryopteris melanocarpa grows in groups on the entrance wall of an artificial cave known to have been built in the early 1900s in Mt. Daeroksan in Gasi-ri, Pyoseom-myeon, Seogwipo-si, Jeju-do. This taxon occurs together with Leptogramma pozoi subsp. mollisima (Fisch. ex Kunze) Nakaike and D. hikonensis (H. Itô) Nakaike, among others, and outside the cave, Eurya japonica Thunb., Miscanthus sinensis Andersson, and Polystichum polyblepharum (Roem. ex Kunze) C. Presl, among others, were growing densely.
Notes: Japanese name of D. melanocarpa is ‘クロミノイタチシダ’ (Kurominoitachishida), which means apricot fern with black spores, and in Taiwan, it is also called ‘黑孢鱗毛蕨’ (Hei-bao-lin-mao-jue) with the same meaning. In Korea, it was also confirmed that mature spores are black, and it was newly named Geom-eun-jok-je-bi-go-sa-ri.
Key to D. melanocarpa and related taxa in Korea
1. Gemma present at basal of stipe ······················· D. sabaei
1. Gemma absent at basal of stipe.
2. Glandular hairs absent on stipe and costa; lamina 2-pinnate; matured indusium irregularly lacerate ··········· ································································· D. subexaltata
2. Glandular hairs present on stipe and costa; lamina 2–3-pinnate; matured indusium entire.
3. Stipe scales lanceolate; lamina lanceolately ovate, subpapery ················································· D. sparsa
3. Stipe scales broadly lanceolate; lamina triangular lanceolate, herbaceous ·················· D. melanocarpa
Discussion
Dryopteris melanocarpa was initially discovered in the Arisan area in central Taiwan by Hayata and Sasaki. According to the original article, it shares morphological features with D. sabaei, such as the center of the indusium that is also elevated, but it can be distinguished by its much more pointed pinna. While it exhibits the closest morphological resemblance to D. sparsa, it is clearly distinguishable by the longer pinnules on the lowest pinna. Its scales are ovate and gradually become narrower towards the upper part. The lamina is recorded as being ovate, measuring 34 cm in length and 25 cm in width, and is described to be 2-pinnate to 2- pinnatifid. However, both type specimens clearly show the presence of a costule on the lowest pinna, indicating a 3-pinnate lamina. The description of 2-pinnate to 2-pinnatifid on the lowest pinna can be considered to be referring to a 3- pinnate lamina (Hayata, 1914). According to the publication Flora of China, basal stipe scales are ovate, and the lamina is also ovate and 2-pinnate to 3-pinnatifid (eFlora, 2008). Darnaedi et al. (1989) reported the scales to be ovatelanceolate and the lamina to be triangular or pentagonal, tapering abruptly towards the apex, describing it to be 3- pinnate or 3-pinnatifid. This confirms that after the original article, subsequent literature has recorded it as 3-pinnate or higher. In Korean-type specimens, the length of the lamina is in the range of 15–20 cm, and the width ranges from 8.5 to 15 cm, showing an overall smaller size compared to the types described in the original article, indicating differences in size and the lamina shape (Table 1).
In Japan, it was reported that the pinnules of D. sparsa measure 2.1–2.5 cm in length, while those of D. melanocarpa range from 1.8 to 2.2 cm (Ebihara, 2017). Both species were described as having almost no glandular hairs on the stipes (Ebihara, 2017). However, Korean specimens show the presence of glandular hairs on the stipes (Fig. 1). D. sparsa is endemic to Mulchatoreum in Jeju-si and Gungdaeak in Seogwipo-si, with individuals from the Seogwipo region tending to be larger in size, although the diagnostic characters are identical. The longer pinnules observed in D. sparsa compared to those in D. melanocarpa, as indicated in Table 1, can be attributed to the larger overall lamina size of the individuals found in Gungdaeak.
Dryopteris subexaltata is characterized by lanceolate basal stipe scales, 2-pinnate lamina, and with irregularly divided indusium. Dryopteris hayatae, reported in Japan, shares similar overall morphological features with D. subexaltata but is recognized as a new species due to the presence of glandular hairs on the indusium (Tagawa, 1932). Some researchers consider it synonymous with D. subexaltata (Moon et al., 2002), but other researchers recognizes D. hayatae as the correct name (Ebihara, 2017).
Dryopteris melanocarpa Hayata var. elegans Seriz. is distributed on Yakushima Island. It is known to have a larger and deeply divided lamina and longer pinna stalks compared to those of the main species (Ebihara, 2017). The publication Index to Ferns and Lycophytes of Japan (Ebihara and Kasetani, 2018) considers it a synonym of D. melanocarpa.
Acknowledgements
We thank Professor Yong-In Kim of Hallym University and Dr. Sang-Jun Lee of the National Institute of Biological Resources for their assistance in this study. This study was conducted with support from the Jeju National University Basic Science Research Center.
Notes
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.