동소적으로 서식하는 은대난초속 3종(난과)의 미세 공간 분포에 대한 참고 사항: 종의 경계 유지에 대한 암시
Notes on fine-scale spatial distribution of three Cephalanthera species (Orchidaceae) that grow in sympatry in Korea: Implications for maintenance of species boundaries
Article information
Abstract
적 요
자가화합성이고 3종의 육상 난초인 은난초, 금난초, 은대난초가 간혹 동소적 개체군들에서 동시에 개화한 다. 흰꽃이 완전히 열리지 않는 은난초와 은대난초는 주로 자가수분하며, 밝은 노란색 꽃이 완전히 열리는 금난초 는 꽃 내 먹이가 존재하는 듯 속이는 방법으로 곤충을 유인하면서 타가교배한다. 잡종의 형성은 강한 수분 장벽(꽃 간의 구조적 격리) 때문에 종간에 거의 일어나지 않는다. 만약 이 들 3종이 종간 잡종화의 방지로 진화하였다면, 저 자들은 3 종의 공간 분포 양상이 서로 공간적으로 분리(즉, ‘공간적 반발’) 될 것으로 기대할 수 있다. 이 예측을 검 증하기 위해 한국의 경상남도 연화산군립공원 내 동소적집단에서 동시개화하는 3 종류의 은대난초 종의 공간적 분포를 연구하였다. 각각 개체군에서는 개체들이 공간적으로 강하게 뭉쳐 있으나, 종 간에서는 이들이 공간적으로 서로 독립되어 있는 결과는 다른 종 들에서 보여준 기존 연구와 다르다. 저자들은 이들 은대난초속 종이 유사한 균 근 형성 균류들을 공유함으로써 공간적으로 다소 무작위로 분포 해왔을 것이라는 가정을 제시한다.
Trans Abstract
The three self-compatible, terrestrial orchids Cephalanthera erecta, C. falcata, and C. longibracteata flower synchronously in sympatric populations. Cephalanthera erecta and C. longibracteata, which have white flowers that do not fully open, are predominantly autogamous, whereas the food-deceptive C. falcata, whose bright yellow flowers open completely, is predominantly outcrossing. The formation of hybrids rarely occurs between species owing to strong pre-pollination barriers (floral isolation). If these three species have evolved toward the prevention of interspecific hybridization, we can expect that the spatial distribution patterns of the three species would be characterized as spatial segregation (i.e., ‘spatial repulsion’) from each other. To test this prediction, we studied the three Cephalanthera species in sympatric populations showing coincident flowering within Yeonwhasan Provincial Park (YPP, Gyeongsangnam Province, South Korea). We found strong spatial aggregation in each population and spatial independence in the interspecific spatial distribution, differing from previous studies. We further hypothesize that Cephalanthera species in sympatry within YPP are distributed somewhat randomly in space, perhaps due to the sharing of similar mycorrhizal fungi.
Spatial pattern (i.e., distribution of individuals in space) is an important characteristic of plant populations, as it may have dramatic effects on population dynamics, performance, reproductive success, viability, and plant evolution in general. For example, interspecific hybridization may be impeded by the nonrandom spatial distribution of individuals within sympatric populations. There is a growing consensus that strong spatial aggregation is the rule in most natural terrestrial orchid populations (Chung et al., 2004, 2005; Jacquemyn et al., 2009; McCormick et al., 2009, 2012), perhaps due to limited seed dispersal and patchy distributions of orchid mycorrhizal fungi (Jersáková and Malinová, 2007; Rasmussen and Rasmussen, 2009; Jacquemyn et al., 2014). As the distribution of pollen dispersal distances within populations of terrestrial orchids is in general leptokurtic (e.g., Peakall, 1989; Chung and Chung, 2015), we would expect that formation of interspecific hybrids in food-deceptive orchids would be much easier between closely spaced orchid species than distantly spaced ones. For example, the closely related and easily hybridizing food-deceptive orchids Platanthera aquilonis Sheviak/P. dilatata (Pursh) Lindl. ex L. C. Beck and Orchis purpurea Huds./O. militaris L. are spatially positively associated (i.e., ‘spatial attraction’) when occur in sympatry (Wallace, 2006; Jacquemyn et al., 2012a). However, many congeneric orchid species grow sympatrically and do not hybridize because of differences in pollination syndromes. In addition, sympatric congeners of plant species are common and the species boundaries of most sympatric congeners are maintained without hybridization.
The three non-clonal, terrestrial orchid species Cephalanthera erecta (Thunb.) Blume, C. falcata (Thunb.) Blume, and C. longibracteata Blume often occur in sympatry in southern Korea. Cephalanthera erecta and C. longibracteata, which have white flowers that do not fully open, are predominantly autogamous, whereas the food-deceptive C. falcata, whose bright yellow flowers open completely, is predominantly outcrossing (Tanaka, 1965; Suetsugu et al., 2015). Hybridization rarely occurs between the three Cephalanthera species owing to strong pre-pollination barriers; there is no morphological or physiological intermediacy, or known hybrid zones or derivative hybrid species (Lee and Kim, 1986). Furthermore, allozyme data in sympatry unequivocally show lack of interspecific hybrids in several populations in southern Korea, suggesting that the species likely have been diverging for quite some time (Chung et al., unpubl. data).
The relationships between the spatial distribution of orchid congeners in sympatry and the hybridization amplitude, and maintenance of species boundaries are not well understood. If the three Cephalanthera species have evolved towards prevention of interspecific hybridization, we may also expect that spatial distribution patterns of the three species would be spatially segregated (i.e., ‘spatial repulsion’) from each other (e.g., Jacquemyn et al., 2014). To test this prediction, we studied the spatial distribution of individuals in populations of the three Cephalanthera species that show coincident flowering in sympatric populations within Yeonwhasan Provincial Park (YPP), located in Gyeongsangnam Province of South Korea (Fig. 1).
Materials and Methods
Study species
Cephalanthera erecta is 20–40 cm high, with 3–10 flowers per inflorescence. This species is widely distributed in China, Korea, and Japan, including Hokkaido (Kitamura et al., 1986). In South Korea, C. erecta grows in humus soils under broadleaved or deciduous pine-oak forests mainly in the southeastern corner of the country (M. Y. Chung and M. G. Chung, pers. obs.). White flowers bloom during May and June. The first bract length is variable within species (1.5–7 cm long) (Lee and Kim, 1986). The pollinia of C. erecta are in contact with the upper margin of the stigma situated below them (Tanaka, 1965). Thus, C. erecta is also highly self-compatible, and autogamy is the dominant mating strategy (M. Y. Chung and M. G. Chung, pers. obs.; K. Suetsugu, pers. comm.). High fruit set (92–95%) for C. erecta in dense forest understories may reflect capability for self-pollination (M. Y. Chung and M. G. Chung, pers. obs.). Flowers (without odor) only open partially (open from one-third to half) (Lee and Kim, 1986; M. Y. Chung and M. G. Chung, pers. obs.). The chromosome number is 2n = 34 (Lee and Kim, 1986).
Its congener C. falcata is 40–70 cm high, with 3–12 bright yellow flowers per raceme that bloom from late April to late June. This species is also widely distributed in China, Korea, and Japan (Kitamura et al., 1986). It grows sparsely at the edges or on the understory of broadleaved or pine-oak forests (M. Y. Chung and M. G. Chung, pers. obs.). The length of the first bracts (0.2–0.8 cm long) (Lee and Kim, 1986) is significantly shorter than that for C. erecta and C. longibracteata. As the species is food-deceptive (non-rewarding), this coloration could function in the mimicry of specific rewarding plants (Suetsugu et al., 2015). Flowers, which have faint but sweet scent, open fully (Lee and Kim, 1986; Suetsugu et al., 2015; M. Y. Chung and M. G. Chung, pers. obs.). The location of the pollinia bounded by the upper margin of stigma was suggested to be an adaptation for preventing autonomous attachment to the stigmatic surface of the same flower (Tanaka, 1965). Previous pollination experiments in Japan demonstrated that C. falcata is self-compatible and neither autogamous nor apogamous, but is strongly pollinator (the andrenid bee Andrena aburana: Andrenidae) dependent (Suetsugu et al., 2015; Ito et al., 2016). The chromosome number is also 2n = 34 (Kitamura et al., 1986; Lee and Kim, 1986).
Finally, C. longibracteata is 30–50 cm tall, with 3–12 flowers per inflorescence. The species grows in the warmer parts of southern Korea and in central and southern Japan (Kitamura et al., 1986). In South Korea, C. longibracteata grows in humus soils under deciduous pine-oak forests mainly in the eastern and southeastern parts of the country, including Jeju Island (M. Y. Chung and M. G. Chung, pers. obs.). Like C. erecta, the length of the first bracts is also variable within species (4–12 cm long) (Lee and Kim, 1986). The relatively small (ca. 1.0 cm long) white flowers bloom in May and June. Like C. erecta, flowers, without odor, do not fully open (open from one-third to half) (Lee and Kim, 1986). Owing to the close morphology between C. longibracteata and C. erecta (white flowers, 2–5 mm long labellum, and 5–12 mm long, lanceolate petals) (Lee and Kim, 1986), it has been often difficult to identify species with confidence (Lee and Kim, 1986; M. Y. Chung and M. G. Chung, pers. obs.). In YPP, we only observed a small bee (Lasioglossum sp.: Halictidae) visiting flowers (but the bee did not enter inside the labellum) of C. longibracteata. Like C. erecta, the pollinia of C. longibracteata are in contact with the upper edge of the stigma (Tanaka, 1965). Cephalanthera longibracteata is also highly self-compatible and predominantly autogamous (K. Suetsugu, pers. comm.). We have observed high fruit-set (ca. 98%) in a pollinator-free screened greenhouse (M. Y. Chung and M. G. Chung, unpubl. data). The chromosome number is 2n = 32 (Lee and Kim, 1986).
In southern Korea, Cephalanthera erecta grows together with C. falcata and C. longibracteata in a few sympatric populations (including Geojae Island, Namhae Island, and YPP), where the density of C. erecta is often low (the number of individuals per population is often below 50) compared to the other two species.
Study populations
To reveal the patterns of spatial distribution of each species and between species, we mapped samples from all the individuals that were present within a series of small sympatric populations at the landscape level in YPP (600 × 600-m area [36 ha]) (Figs. 1, 2): YPP-1 (C. falcata, n = 50), YPP-2 (C. falcata, n = 49; C. erecta, n = 1), YPP-3 (C. falcata, n = 19; C. erecta, n = 20; C. longibracteata, n = 13), and YPP-4 (C. falcata, n = 23; C. longibracteata, n = 14). Although YPP-1 consisted of only C. falcata, we included it because of spatial proximity with the other three populations (Fig. 1). Species in each population were identified based on flower color and length of the first bracts (Kitamura et al., 1986), though the latter character was variable and overlapped between C. erecta and C. longibracteata (3–8 cm, C. erecta; 4–12 cm, C. longibracteata) (M. Y. Chung and M. G. Chung, unpubl. data).
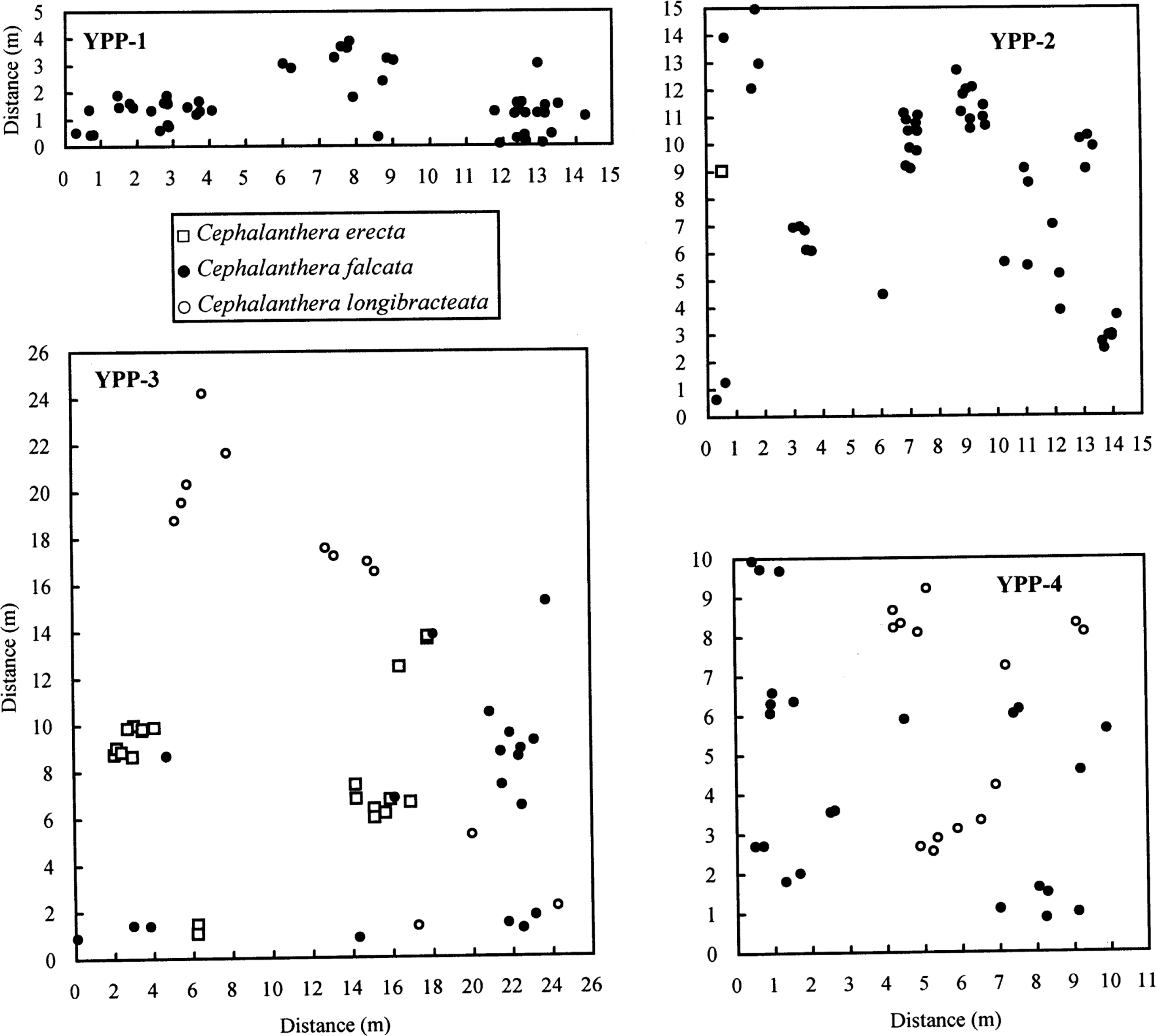
Spatial distribution of individuals within the four populations (YPP-1 toYPP-4) in Yeonwhasan Provincial Park: YPP-1 (C. falcata, n = 50), YPP-2 (C. falcata, n = 49; C. erecta, n = 1), YPP-3 (C. erecta, n = 20; C. falcata, n = 19; C. longibracteata, n = 13), and YPP-4 (C. falcata, n = 23; C. longibracteata, n = 14).
Spatial distribution of individuals
1. Univariate analysis
To assess the spatial distribution of mapped individuals of the three Cephalanthera species in the four populations in which one to three species occur (Fig. 2), we calculated the univariate O-ring statistic O11(r) of Wiegand and Moloney (2004) from the mean number of individuals in an annulus of radius (r) around each plant. We plotted against the spatial scale r at starting ring width 0.5 m with a 0.5-m lag. Since the use of ring widths greater than half the shortest plot side introduces bias due to edge effects, the maximal ring width was set at less than half the shortest plot width (Haase, 1995). We used the common null model of ‘complete spatial randomness (CSR)’ distribution (null intraspecific interaction), where any point (i.e., a plant individual of a given species) of the pattern examined has an equal probability of occurring at any position in the mapped area, and the position of a given individual is independent of the position of any other individual of the same species (i.e., no interaction) (Wiegand and Moloney, 2004). For reference to the point pattern expected under CSR, the first order intensity, λ, was calculated. For each study population, 95% confidence intervals (CIs) about CSR (i.e., λ) for a given r were constructed from the 25th and 975th highest of the ordered O11(r) from 999 replicates by Monte Carlo simulation (Wiegand et al., 2000). An observed value of O11(r) outside of this envelope was judged as a significant departure from CSR, with an observed value above, within, or below the envelope indicating spatial clumping, spatial randomness, or spatial regularity (hyper-dispersion), respectively, at radius r (Diggle, 1983). All calculations and simulations were conducted using the Programita software package (Wiegand, 2003).
2. Bivariate analysis
We tested spatial independence between the three Cephalanthera species using the bivariate O-ring statistic O12(r) of Wiegand and Moloney (2004) with an independence (a ‘toroidal shift’) null model. The pattern of a given species remains fixed whereas the pattern of the second species is randomly shifted as a whole across the study area, using a torus (Wiegand et al., 2006). This analysis is applied to plant populations with finite size and irregular shape (e.g., Almirón and Martínez Carretero, 2015). We tested for independence of the eight possible bivariate patterns (six in YPP-3; two in YPP-4). Like the univariate analysis, 95% CIs about the null model of independence for a given r were constructed from the 25th and 975th highest of the ordered O12(r) from 999 replicates by Monte Carlo simulation (Wiegand et al., 2000). An observed value of O12(r) outside CIs was judged as a significant departure from independence, with an observed value above, within, or below the envelope indicating spatial attraction, spatial independence, or spatial repulsion, respectively, at radius r (Diggle, 1983). These analyses were carried out using the grid-based estimators in Programita.
Results
O11(r) function analyses of each of the seven populations assigned to the three Cephalanthera species showed significant spatial aggregation of individuals. Individuals of C. falcata (YPP-4) and C. longibracteata (YPP-3 and YPP-4) were significantly aggregated between 0–0.5 m; C. falcata (YPP-1 and YPP-2) between ca. 0–1 m; C. erecta (YPP-3) between 0–1.5 m; and C. falcata (YPP-3) between ca. 0–2 m (Fig. 3).

The univariate O-ring statistic O11(r) at different scales r with upper and lower confidence envelopes representing the 25th lowest and highest values of 999 Monte Carlo simulations with the null hypothesis of complete spatial randomness (CSR). If O11(r) is above the upper confidence interval, then the pattern is significantly aggregated; if O11(r) is below the lower confidence interval, the pattern is significantly regular at the considered scale (p < 0.05). All analyses were performed with a cell size of 0.5 × 0.5 m. The first-order intensity, λ, of the point pattern within populations was 0.24 in C. falcata (YPP-1), 0.06 in C. falcata (YPP-2), 0.022 in C. erecta (YPP-3), 0.013 C. falcata (YPP-3), 0.007 in C. longibracteata (YPP-3), 0.067 in C. falcata (YPP-4), and 0.10 in C. longibracteata (YPP-4). Note that the scales x and y axes differ among panels.
The interspecific spatial distribution (species–species spatial associations) was not significantly different from the null model of spatial ‘independence’ (Fig. 4), as the empirical bivariate O12(r) function fell within the 95% CIs (Fig. 4). Although the distribution between C. falcata and C. longibracteata in YPP-4 appeared to show a spatial repulsion between 0–2 m, this pattern was not significantly different from spatial independence (Fig. 4). We found very similar results from other four possible bivariate patterns (three in YPP-3; one in YPP-4) by fixing the second orchid species (e.g., C. falcata vs. C. erecta in YPP-3 rather than C. erecta vs. C. falcata shown in Fig. 4) (data not shown).
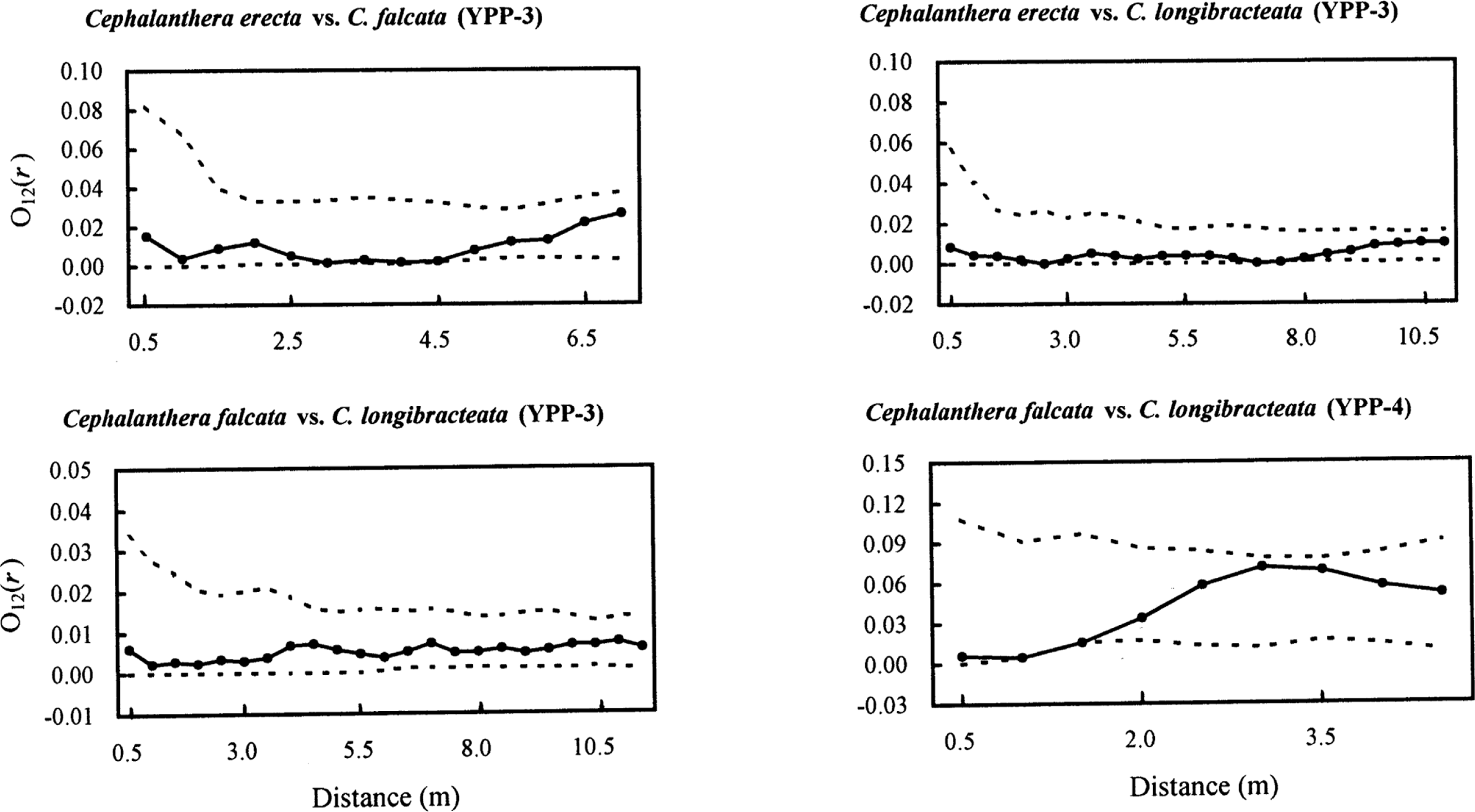
The bivariate O-ring statistic O12(r) at different scales r. Confidence envelopes were based on the 25th lowest and highest values of 999 Monte Carlo simulations with an independence (the toroidal-shift) null model. If O12(r) is above the upper confidence interval, then the two processes display significant attraction; if O12(r) is below the lower confidence interval, they show significant repulsion (p < 0.05). Note different x and y axes among panels.
Discussion
As seeds in orchids rely heavily on mycorrhizal fungi for successful germination (Smith and Read, 2008; Rasmussen and Rasmussen, 2009), their recruitment and establishment tend to be sensitive to abundance of mycorrhizal fungi and their spatial distribution (McCormick et al., 2009; Jacquemyn et al., 2014). In some orchid species, successful seed germination tends to be negatively correlated with the distance from the maternal plants, but others show no such relationship (McKendrick et al., 2000). These studies suggested complex processes in associations between terrestrial orchids and their accompanying mycorrhizal fungi.
Our results revealed that the three Cephalanthera species showed significant spatial aggregation of individuals within very short distances (of ca. 0–2 m), which gives support to the general trend of strong spatial aggregation in most natural terrestrial orchid populations. The fact that orchids are not evenly distributed in a population results perhaps from limited seed dispersal (Jersáková and Malinová, 2007; Jacquemyn et al., 2009), patchy distributions of orchid mycorrhizal fungi (McCormick et al., 2009, 2012; Rasmussen and Rasmussen, 2009; Jacquemyn et al., 2012b, 2014), and microhabitat heterogeneity (e.g., light, soil moisture, competition with other plant species).
We predicted that spatial distribution patterns of the three species would be spatially segregated (i.e., ‘spatial repulsion’) (Jacquemyn et al., 2014) from each other to prevent interspecific hybridization. However, interspecific spatial distribution was not significantly different from the null model of spatial independence (i.e., no spatial attraction or repulsion) in our study system.
To date, there is no consistent trend in interspecific association between food-deceptive orchids occurring in sympatric populations. In South Korea, Chung et al. (2005) investigated patterns of hybridization in two food-deceptive Liparis species. The authors found, using Ripley’s K statistic, that the parental species (Liparis kumokiri F. Maekawa and L. makinoana Schltr.) were spatially segregated (i.e., spatial repulsion), but the spatial distribution of hybrids was associated with only one of the parental species (L. kumokiri). More recently, Jacquemyn et al. (2012a) found a significantly positive association between the predominantly hybridizing species Orchis purpurea and O. militaris in sympatry. Jacquemyn et al. (2012b), however, reported significant spatial segregation among the three food-deceptive terrestrial orchids Anacamptis morio (L.) R. M. Bateman, Pridgeon & M. W. Chase, Gymnadenia conopsea (L.) R. Br., and Orchis mascula (L.) L. in sympatry. Similarly, Jacquemyn et al. (2014) tested spatial independence of seven food-deceptive terrestrial orchids co-occurring in 25 × 25 m plots in two Mediterranean grasslands and found strong spatial segregation due to distinctive mycorrhizal communities. Based on these results, the authors suggested that ecological factors such as mycorrhizal fungus specificity is a primary driving force for shaping a sort of niche partitioning in terrestrial orchids, resulting in orchid coexistence.
As described above, there is considerable variation in spatial species–species associations in orchids in sympatry ranging from spatial attraction to spatial repulsion. This suggests that the differences in the extent of seed dispersal, kinds and composition of mycorrhizal fungal community, and the patterns and extent of spatial distribution of dominant fungal lineages might determine spatial distribution of coexisting terrestrial orchid species in general (Jacquemyn et al., 2012a, 2014).
Taken these all, somewhat random species–species associations between/among the three Cephalanthera species might be attributed to sharing the same or similar mycorrhizal fungi by forming similar mycorrhizal communities. To test this hypothesis, detailed studies on the identification of mycorrhizal fungi, their spatial distribution, and, more specifically, determination of the major role of mycorrhizal fungi in affecting orchid coexistence are necessary.
Finally, we think that it is necessary to provide an alternative explanation. As mentioned before, the three species can be classified as autogamous vs. allogamous and are differentiated at the chromosome level (2n = 34 for C. erecta and C. falcata, 2n = 32 for C. longibracteata). One may speculate that, because such reproductive barriers have already been acquired in sympatry, spatial separation between species might not be necessary.
Acknowledgements
This study is supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A2B4012215) to M.G.C. Prof. Masayuki Maki and Dr. Tomohisa Yukawa kindly translated a Cephalanthera breeding system reference written in Japanese into English.